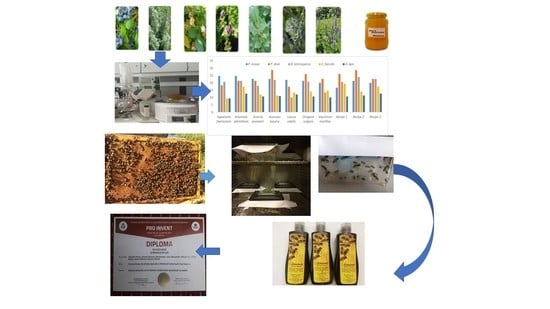
Biologically Active Extracts from Different Medicinal Plants Tested as Potential Additives against Bee Pathogens
Abstract
:1. Introduction
2. Results
2.1. Total Phenolic, Flavonoid Content and Phenolic Compounds
2.2. Antioxidant and Antimicrobial Activity
2.3. Medicinal Additive Recipes
2.4. Effectivity against N. ceranae
3. Discussion
4. Materials and Methods
4.1. Plant Material and Ethanolic Extracts
4.2. Total Phenolic, Flavonoid Content and Polyphenolic Composition
4.3. Antioxidant Activity
4.4. Antimicrobial Activity
4.5. Nosema Infection Assay
4.6. Determination of Spore Levels
4.7. DNA Extraction and Species Verification
4.8. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebeling, J.; Knispel, H.; Hertlein, G.; Fünfhaus, A.; Genersch, E. Biology of Paenibacillus larvae, a deadly pathogen of honey bee larvae. Appl. Microbiol. Biotechnol. 2016, 100, 7387–7395. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E.; Locke, B.; Sircoulomb, F.; Schäfer, M.O. Bacterial Diseases in Honeybees. Curr. Clin. Microbiol. Rep. 2018, 5, 18–25. [Google Scholar] [CrossRef]
- Erler, S.; Lewkowski, O.; Poehlein, A.; Forsgren, E. The Curious Case of Achromobacter eurydice, a Gram-Variable Pleomorphic Bacterium Associated with European Foulbrood Disease in Honeybees. Microb. Ecol. 2018, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Human, H.; Pirk, C.W.W.; Crewe, R.M.; Dietemann, V. The honeybee disease American foulbrood—An African perspective. Afr. Entomol. 2011, 19, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E.; Budge, G.E.; Charrière, J.D.; Hornitzky, M.A.Z. Standard methods for European foulbrood research. J. Apic. Res. 2013, 52, 1–14. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Aronstein, K.A.; Murray, K.D. Chalkbrood disease in honey bees. J. Invertebr. Pathol. 2010, 103, S20–S29. [Google Scholar] [CrossRef]
- Paşca, C.; Mărghitaş, L.A.; Șonea, C.; Bobiş, O.; Buzura-Matei, I.A.; Dezmirean, D.S. A Review of Nosema cerane and Nosema apis: Characterization and Impact for Beekeeping. Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2019, 76, 77–87. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Maes, P.; Anderson, K.E. The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE 2014, 9, e95056. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.J.; Al-Ghamdi, A.; Usmani, S.; Al-Waili, N.; Nuru, A.; Sharma, D.; Ali Khan, K.; Kaurd, M.; Omer, M. In vitro evaluation of the effects of some plant essential oils on Paenibacillus larvae, the causative agent of American foulbrood. Biotechnol. Biotechnol. Equip. 2016, 30, 49–55. [Google Scholar] [CrossRef]
- Fernández, N.J.; Damiani, N.; Podaza, E.A.; Martucci, J.F.; Fasce, D.; Quiroz, F.; Meretta, P.E.; Quintana, S.; Eguaras, M.J.; Gende, L.B. Laurus nobilis L. Extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudi J. Biol. Sci. 2019, 26, 906–912. [Google Scholar] [CrossRef]
- Collins, W.; Lowen, N.; Blake, D.J. Caffeic Acid Esters Are Effective Bactericidal Compounds Against Paenibacillus larvae by Altering Intracellular Oxidant and Antioxidant Levels. Biomolecules 2019, 9, 312. [Google Scholar] [CrossRef] [Green Version]
- Chantawannakul, P.; Puchanichanthranon, T.; Wongsiri, S. Inhibitory Effects of Some Medicinal Plant Extracts on the Growth of Ascosphaera apis. Acta Hort. 2005, 4, 183–189. [Google Scholar] [CrossRef]
- Fries, I.; Feng, F.; Silva, A.D.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n.sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis melllifera). J. Invertebr. Pathol. 2010, 103(S), S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hernandez, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailon, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.R.; Shafer, A.B.A.; Rogers, R.E.L.; Shutler, D.; Stewart, D.T. First detection of Nosema ceranae, a microsporidian parasite of European honey bees (Apis mellifera), in Canada and central U.S.A. J. Invertebr. Pathol. 2008, 97, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martin-Hernandez, R.; Botias, C.; Bailon, E.G.; Gonzalez-Porto, A.V.; Barrios, L.; Del Nozal, D.J.; Bernal, J.L.; Jimenez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2668. [Google Scholar] [CrossRef]
- Guo, R.; Chen, D.; Xiong, C.; Hou, C.; Zheng, Y.; Fu, Z.; Liang, Q.; Diao, Q.; Zhang, L.; Wang, H.; et al. First identification of long non-coding RNAs in fungal parasite Nosema ceranae. Apidologie 2018, 49, 660–670. [Google Scholar] [CrossRef] [Green Version]
- McGowan, J.; De la Mora, A.; Goodwin, P.H.; Habash, M.; Hamiduzzaman, M.M.; Kelly, P.G.; Guzman-Novoa, E. Viability and infectivity of fresh and cryopreserved Nosema ceranae spores. J. Microbiol. Methods 2016, 131, 16–22. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Roy. Soc. Lond. B. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Chaimanee, V.; Thongtue, U.; Sornmai, N.; Songsri, S.; Pettis, J.S. Antimicrobial activity of plant extracts against the honeybee pathogens, Paenibacillus larvae and Ascosphaera apis and their topical toxicity to Apis mellifera adults. J. Appl. Microbiol. 2017, 123, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Gochnauer, T.A. Drugs fight foulbrood disease in bees. Minn. Home Fam. Sci. 1951, 9, 15. [Google Scholar]
- Genersch, E. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, 10–19. [Google Scholar] [CrossRef]
- McCowen, M.C.; Callender, M.E.; Lawlis, J.F. Fumagillin (H-3), a new antibiotic with amebicidal properties. Science 1951, 113, 202–203. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef] [Green Version]
- Mutinelli, F. European legislation governing the authorisation of veterinary medical products with particular reference to the use of drugs for the control of honey bee diseases. Apiacta 2003, 38, 156–168. [Google Scholar]
- Alonso-Salces, R.M.; Cugneta, N.M.; Guapari, E.; Pellegrini, M.C.; Aubone, J.; de Piano, F.G.; Antues, K.; Fuselli, R. Natural strategies for control of Paenibacillus larvae, the causal agent of American foulbrood in honey bees: A review. Apidologie 2017, 48, 387–400. [Google Scholar] [CrossRef]
- Tauber, J.P.; Collins, W.R.; Schwarz, R.S.; Chen, Y.; Grubbs, K.; Huang, Q.; Lopez, D.; Peterson, R.; Evans, J. Natural Product Medicines for Honey Bees: Perspective and Protocols. Insects 2019, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Damiani, N.; Fernández, N.J.; Porrini, M.P.; Gende, L.B.; Álvarez, E.; Buffa, F.; Brasesco, C.; Maggi, M.D.; Marcangeli, A.J.; Eguaras, M.J. Laurel leaf extracts for honeybee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Aurori, A.C.; Bobiş, O.; Dezmirean, D.S.; Mărghitaş, L.A.; Erler, S. Bay laurel (Laurus nobilis) as potential antiviral treatment in naturally BQCV infected honeybees. Virus Res. 2016, 222, 29–33. [Google Scholar] [CrossRef]
- Calderone, N.W.; Shimanuki, H.; Allen-Wardell, G. An in vitro evaluation of botanical compounds for the control of the honey bee pathogens Bacillus larvae and Ascosphaera apis, and the secondary invader Bacillus alvei. J. Essent. Oil Res. 1994, 6, 279–287. [Google Scholar] [CrossRef]
- Saeed, S.; Tariq, P. Antibacterial activity of oregano (Origanum Vulgare Linn.) against gram positive bacteria. Pak. J. Pharm. Sci. 2009, 22, 421–424. [Google Scholar]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease associated microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernklau, E.; Bjostad, L.; Hogeboom, A.; Carlisle, A.H.S.A. Dietary Phytochemicals, Honey Bee Longevity and Pathogen Tolerance. Insects 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Ptaszynska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef] [PubMed]
- Arismendi, N.; Vargas, M.; López, D.M.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Dumitru, A.; Chioveanu, G.; Ionita, M.; Dobre, G.; Mitrea, I.L. “In Vitro” Studies On Using Natural Essential Oils In Treatment Of Nosemosis In Honey Bees: Determination Of The Therapeutic Dose, Scientific Works. Series C. Vet. Med. 2017, 63, 165–170. [Google Scholar]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Al, K.F.; Chernyshova, A.M.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J. 2020, 14, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Lamas, A.; Arteaga, V.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Antimicrobial Activity of Five Apitoxins from Apis mellifera on Two Common Foodborne Pathogens. Antibiotics 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Terentjeva, M.; Žiarovská, J.; Kowalczewski, P.Ł. In Vitro Antagonistic Effect of Gut Bacteriota Isolated from Indigenous Honey Bees and Essential Oils against Paenibacillus Larvae. Int. J. Mol. Sci. 2020, 21, 6736. [Google Scholar] [CrossRef]
- Hassona, N.M.K. Using natural products to control foulbrood diseases in honey bee Apis mellifera L. colonies under egyptian conditions. Menoufia J. Plant. Prot. 2017, 2, 153–165. [Google Scholar] [CrossRef]
- Wilson, M.B.; Brinkman, D.; Spivak, M.; Gardner, G.; Cohen, J.D. Regional variation in composition and antimicrobial activity of US propolis against Paenibacillus larvae and Ascosphaera apis. J. Invertebr. Pathol. 2015, 124, 44–50. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Marghitas, L.A.; Chirila, F.; Copaciu, F.; Simonca, V.; Bobis, O.; Erler, S. Influence of geographic origin, plant source and polyphenolic substances on antimicrobial properties of propolis against human and honey bee pathogens. J. Apic. Res. 2017, 56, 588–597. [Google Scholar] [CrossRef]
- Cristina, R.T.; Kovacevic, Z.; Cincovic, M.; Dumitrescu, E.; Muselin, F.; Imre, K.; Militaru, D.; Mederle, N.; Radulov, I.; Hădărugă, N.; et al. Composition and Efficacy of a Natural Phytotherapeutic Blend against Nosemosis in Honey Bees. Sustainability 2020, 12, 5868. [Google Scholar] [CrossRef]
- Chioveanu, G.; Ionescu, D.; Mardare, A. Control of nosemosis–the treatment with Protofil. Apiacta 2004, 39, 31–38. [Google Scholar]
- Gajger, I.T.; Petrinec, Z.; Pinter, L.; Kozarić, Z. Experimental Treatment of Nosema Disease with “Nozevit” Phyto-pharmacological Preparation. J. Apic. Res. 2009, 149, 485–490. [Google Scholar]
- Balamurugan, R.; Park, J.K.; Lee, J.K. Anti-nosemosis activity of phenolic compounds derived from Artemisia dubia and Aster scaber. J. Apic. Res. 2020, 59, 1–11. [Google Scholar] [CrossRef]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Berbeć, E.; Migdał, P. Herbal extracts in nosemosis treatment in honeybee (Apis mellifera L.). In The Book of Articles National Scientific Conference “Science and Young Researchers”, 3rd ed.; Promovendi: Łódź, Poland, 2019. [Google Scholar]
- Pașca, C.; Mărghitaș, L.; Dezmirean, D.; Bobiș, O.; Bonta, V.; Chirilă, F.; Matei, I.; Fiț, N. Medicinal Plants Based Products Tested on Pathogens Isolated from Mastitis Milk. Molecules 2017, 22, 1473. [Google Scholar] [CrossRef] [Green Version]
- Stănilă, M.A.; Cioanca, B.; Diaconeasa, Z.; Stănilă, S.; Sima, N.; Sima, R. Phytochemical Composition and Antioxidant Activity of Various Grain Amaranth Cultivars. Not. Bot. Horti. Agrobot. Cluj Napoca 2019, 47, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Porrini, M.P.; Fernández, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Boligon, A.A.; Piana, M.; Brum, T.F.; Freitas, R.B.; Nunes, L.; Pappis, L.; Alves, C.F.S.; Vaucher, R.A.; Santos, R.C.V.; Athayde, M.L. Potential use of Buddleja thyrsoides for control and prevention of American foulbrood disease in honey bees. J. Apic. Sci. 2014, 58, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Boligon, A.A.; de Brum, T.F.; Zadra, M.; Piana, M.; dos Santos Alves, C.F.; Fausto, V.P.; dos Santos Barboza, J.V.; de Almeida Vaucher, R.; Santos, R.C.V.; Athayde, M.L. Antimicrobial activity of Scutia buxifolia against the honeybee pathogen Paenibacillus larvae. J. Invertebr. Pathol. 2013, 112, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Flesar, J.; Havlik, J.; Kloucek, P.; Rada, V.; Titera, D.; Michal, B.; Stropnicky, M.; Kokoska, L. In vitro growth-inhibitory effect of plant-derived extracts and compounds against Paenibacillus larvae and their acute oral toxicity to adult honey bees. Vet. Microbiol. 2010, 145, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Blank, D.E.; Hörnke Alves, G.; Da Silva Nascente, P.; Freitag, R.A.; Cleff, M.B. Bioactive Compounds and Antifungal Activities of Extracts of Lamiaceae Species. J. Agric. Chem. Environ. 2020, 9, 85–96. [Google Scholar] [CrossRef]
- Duda, S.C.; Marghitas, L.A.; Dezmirean, D.; Duda, M.; Margaoan, R.; Bobis, O. Changes in major bioactive compounds with antioxidant activity of Agastache foeniculum, Lavandula angustifolia, Melissa officinalis and Nepeta cataria: Effect of harvest time and plant species. Ind. Crops Prod. 2015, 77, 499–507. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Diaconeasa, Z.; Socaciu, C. Liberation and recovery of phenolic antioxidants and lipids in chokeberry (Aronia melanocarpa) pomace by solid-state bioprocessing using Aspergillus niger and Rhizopus oligosporus strains. LWT Food Sci. Technol. 2018, 87, 241–249. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Clapa, D.; Borsai, O.; Hârta, M.; Bonta, V.; Szabo, K.; Coman, V.; Bobis, O. Micropropagation, Genetic Fidelity and Phenolic Compound Production of Rheum rhabarbarum L. Plants 2020, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.; Markham, K.R. Structure Information from HPLC and On-line Measured Absorption Spectra: Flavones, Flavonols and Phenolic Acids; Coimbra University Press: Coimbra, Portugal, 2007; p. 14. [Google Scholar]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; Mcmahon, D.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with the Microsporidia Nosema ceranae. J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martin-Hernandez, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honey bees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef]
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martin-Hernandez, R.; Bernal, J. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377. [Google Scholar] [CrossRef] [Green Version]
- Gherman, B.I.; Denner, A.; Bobiş, O.; Dezmirean, D.S.; Mărghitaş, L.A.; Schlüns, H.; Moritz, R.F.; Erler, S. Pathogen-associated self-medication behavior in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 2014, 68, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Erler, S.; Denner, A.; Bobis, O.; Forsgren, E.; Moritz, R.F.A. Diversity of honey stores and their impact on pathogenic bacteria of the honeybee, Apis mellifera. Evol. Ecol. 2014, 20, 3960–3967. [Google Scholar] [CrossRef]
- Aurori, C.M.; Dezmirean, D.S.; Mărghitas, L.A.; Moritz, R.F.A. Nosema apis and N. ceranae in Western Honeybee (Apis mellifera)—Geographical Distribution and Current Methods of Diagnosis. Bul. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2011, 68, 63–70. [Google Scholar]
- Mederle, N.; Lobo, M.L.; Morariu, S.; Morariu, F.; Darabus, G.; Mederle., O.; Matos, O. Microscopic and Molecular Detection of Nosema ceranae in Honeybee Apis mellifera L. from Romania. Rev. Chim. 2018, 69, 3761–3772. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef] [PubMed]


| Sample | Total Phenolic Content mg GAE/g DW | Total Flavonoid Content mg QE/g DW |
|---|---|---|
| Agastache foeniculum | 2.882 ± 0.005 c,d | 2.215 ± 0.001 d |
| Artemisia absinthium | 1.831 ± 0.03 d | 30.520 ± 0.002 a |
| Evernia prunastri | 4.574 ± 0.011 c | 2.560 ± 0.007 d |
| Humulus lupulus | 5.344 ± 0.003 c | 2.511 ± 0.001 d |
| Laurus nobilis | 14.856 ± 0.003 b | 5.685 ± 0.09 c |
| Origanum vulgare | 44.672 ± 0.006 a | 20.835 ± 0.02 b |
| Vaccinium myrtillus | 13.241 ± 0.007 b | 5.710 ± 0.005 c |
| Sunflower honey | 3.570 ± 0.05 c,d | 1.890 ± 0.004 d |
| Identified Compound | Agastache foeniculum | Artemisia | Evernia prunastri | Humulus lupulus | Laurus nobilis | Origanum vulgare | Vaccinium myrtillus | Sunflower Honey |
|---|---|---|---|---|---|---|---|---|
| Chlorogenic acid | 49.88 ± 2.37 b* | n.d.** | 1.44 ± 0.07 c* | n.d.** | n.d.** | n.d.** | 312.18 ± 15.24 a* | 2.95 ± 0.15 c* |
| Syringic acid | n.d.** | n.d.** | n.d.** | n.d.** | 120.68 ± 5.80 a* | n.d.** | 76.68 ± 3.88 b* | n.d.** |
| Ferulic acid | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 118.50 ± 5.78 a* | 0.80 ± 0.04 b* |
| Isoquercitrin | 283.22 ± 14.37 c* | 58.53 ± 2.98 e* | n.d.** | 298.87 ± 15.47 c* | 577.15 ± 29.53 a* | 347.90 ± 16.83 b* | 149.59 ± 7.20 d* | n.d.** |
| Quercetin | 117.69 ± 6.603 c* | 389.73 ± 19.48 a* | n.d.** | n.d.** | 419.32 ± 21.19 a* | n.d.** | 159.10 ± 8.03 b* | 5.99 ± 0.29 d* |
| Miricetin | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 10.21 ± 0.51 a* | 1.03 ± 0.05 b* |
| Naringenin | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 0.08 ± 0.00 a* | n.d.** |
| Kaempferol | n.d.** | n.d.** | n.d.** | n.d.** | 7.46 ± 0.38 b* | n.d.** | 0.28 ± 0.01 c* | 17.80 ± 0.90 a* |
| Vanilic acid | n.d.** | n.d.** | 1.91 ± 0.09 a* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** |
| Vanillin | 40.96 ± 1.94 a* | n.d.** | 6.41 ± 0.01 b* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** |
| Rosmarinic acid | n.d.** | n.d.** | 9.45 ± 0.45 b* | n.d.** | n.d.** | 633.85 ± 32.99 a* | n.d.** | n.d.** |
| Crisin | n.d.** | n.d.** | 70.94 ± 3.6 a* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** |
| o-Cumaric acid | n.d.** | n.d.** | 4.48 ± 0.22 a* | n.d.** | n.d.** | n.d.** | n.d.** | 1.46 ± 0.07 b* |
| Acacetin | 52.83 ± 2.61 a* | n.d.** | 7.55 ± 0.36 b* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** |
| Gallic acid | 38.86 ± 1.85 a* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 0.63 ± 0.03 b* |
| Caffeic acid | 20.34 ± 1.00 a* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 2.71 ± 0.13 b* |
| p-OH Cinnamic acid | 9.01 ± 0.45 a* | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** |
| Apigenin | 17.38 ± 0.88 b* n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 93.55 ± 4.72 a* | n.d.** | 0.93 ± 0.04 c* |
| Rutin | n.d.** | 115.71 ± 5.7 c* | n.d.** | 523.25 ± 26.89 b* | 869.21 ± 30.22 a* | n.d.** | n.d.** | 1.24 ± 0.06 d* |
| Epicatechina | n.d.** | n.d.** | n.d.** | 501.29 ± 24.40 a* | 142.82 ± 7.16 b* n.d.** | n.d.** | n.d.** | n.d.** |
| Vitexin 2-o-ramnoside | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 470.45 ± 23.25 a* | n.d.** | n.d.** |
| Sinapic acid | n.d.** | n.d.** | n.d.** | n.d.** | n.d.** | 446.86 ± 21.96 a* | n.d.** | n.d.** |
| Resveratrol | 128.59 ± 6.72 c | n.d.** | n.d.** | n.d.** | 139.2 ± 6.90 c | 75.19 ± 3.63 a* | n.d.** | n.d.** |
| Total phenolic acids | 512.08 ± 25.18 d | 28.77 ± 1.39 d | 33.94 ± 1.64 d | 95.48 ± 4.70 c | 2015.96 ± 97.01 a | 1095.03 ± 52.96 a | 512.02 ± 24.59 b | 2.71 ± 0.14 d |
| Total flavonoids | 563.97 ± 29.08 d | 84.90 ± 4.26 f | 1323.41 ± 68.86 b | 987.09 ± 48.27 c | 319.26 ± 16.50 e | 26.99 ± 1.37 f |
| Sample | Radical Scavenging Activity (% Inhibition) | Total Antioxidant Power, CUPRAC Value (µmol TE/g DW) |
|---|---|---|
| Agastache foeniculum | 78.80 ± 1.09 b* | 17.96 ± 0.36 d* |
| Artemisia absinthium | 97.50 ± 1.05 a* | 20.97 ± 0.51 d* |
| Evernia prunastri | 76.93 ± 0.86 b* | 18.02 ± 0.24 d* |
| Humulus lupulus | 46.95 ± 0.31 b* | 23.85 ± 0.45 c,d* |
| Laurus nobilis | 50.63 ± 0.82 d* | 39.16 ± 0.82 b* |
| Origanum vulgare | 76.92 ± 0.52 d* | 165.59 ± 1.08 a* |
| Vaccinium myrtillus | 61.31 ± 0.71 c* | 30.99 ± 0.21 b,c* |
| Sunflower honey | 46.99 ± 1.11 d* | 22.10 ± 0.40 c,d* |
| Plant Extracts and Recipes | Paenibacillus larvae | Paenibacillus alvei | Brevibacillus laterosporus | Enterococcus faecalis | Ascosphaera apis |
|---|---|---|---|---|---|
| Agastache foeniculum | 24.67 ± 1.15 a | 18.33 ± 2.88 | 20.67 ± 1.15 | 9.33 ± 2.31 | 9.33 ± 1.15 |
| Artemisia absinthium | 24.67 ± 4.62 | 21.33 ± 1.20 | 21.33 ± 1.15 | 17.33 ± 2.31 | 12.67 ± 0.58 |
| Evernia prunastri | 22.67 ± 2.31 | 21.33 ± 1.20 | 18.00 ± 2.00 | 12.00 ± 2.00 | 10.67 ± 1.15 |
| Humulus lupulus | 22.67 ± 4.62 | 28.67 ± 2.31 | 22.00 ± 0.00 | 11.33 ± 2.31 | 11.00 ± 0.00 |
| Laurus nobilis | 22.00 ± 0.00 | 17.33 ± 2.31 | 10.00 ± 0.00 | 13.33 ± 1.15 | 12.00 ± 2.65 |
| Origanum vulgare | 21.33 ± 2.31 | 26.00 ± 2.00 | 20.67 ± 1.15 | 14.00 ± 0.00 | 10.67 ± 1.15 |
| Vaccinium myrtillus | 20.00 ± 0.00 | 22.67 ± 1.20 | 13.33 ± 5.77 | 14.67 ± 2.31 | 10.00 ± 0.00 |
| Recipe 1 | 16.67 ± 4.62 | 26.00 ± 0.00 | 20.67 ± 1.15 | 19.33 ± 1.15 | 10.67 ± 0.58 |
| Recipe 2 | 21.33 ± 2.31 | 28.67 ± 1.20 b | 24.00 ± 0.00 c,d | 14.00 ± 0.00 | 11.33 ± 2.31 |
| Recipe 3 | 20.00 ± 0.00 | 22.67 ± 2.31 | 22.67 ± 2.31 | 17.33 ± 2.31 | 12.67 ± 1.15 |
| Positive control | 20.00 ± 0.00 | 29.33 ± 4.20 | 20.00 ± 0.00 | 20.67 ± 1.15 | 19.33 ± 1.15 e |
| Negative control | 10.00 ± 2.83 | 11.33 ± 1.20 | 10.33 ± 1.53 | 8.50 ± 2.12 | 8.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pașca, C.; Matei, I.A.; Diaconeasa, Z.; Rotaru, A.; Erler, S.; Dezmirean, D.S. Biologically Active Extracts from Different Medicinal Plants Tested as Potential Additives against Bee Pathogens. Antibiotics 2021, 10, 960. https://doi.org/10.3390/antibiotics10080960
Pașca C, Matei IA, Diaconeasa Z, Rotaru A, Erler S, Dezmirean DS. Biologically Active Extracts from Different Medicinal Plants Tested as Potential Additives against Bee Pathogens. Antibiotics. 2021; 10(8):960. https://doi.org/10.3390/antibiotics10080960
Chicago/Turabian StylePașca, Claudia, Ioana Adriana Matei, Zorița Diaconeasa, Ancuța Rotaru, Silvio Erler, and Daniel Severus Dezmirean. 2021. "Biologically Active Extracts from Different Medicinal Plants Tested as Potential Additives against Bee Pathogens" Antibiotics 10, no. 8: 960. https://doi.org/10.3390/antibiotics10080960
APA StylePașca, C., Matei, I. A., Diaconeasa, Z., Rotaru, A., Erler, S., & Dezmirean, D. S. (2021). Biologically Active Extracts from Different Medicinal Plants Tested as Potential Additives against Bee Pathogens. Antibiotics, 10(8), 960. https://doi.org/10.3390/antibiotics10080960