Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies
Abstract
:1. Introduction
2. Biofilms in SSIs
2.1. Biofilm-Forming Bacteria Associated with SSIs
2.2. Biofilm Recalcitrance to Antimicrobial Treatments
3. Prevention of SSIs
4. Conventional Treatment and Management of Biofilm-Associated SSIs
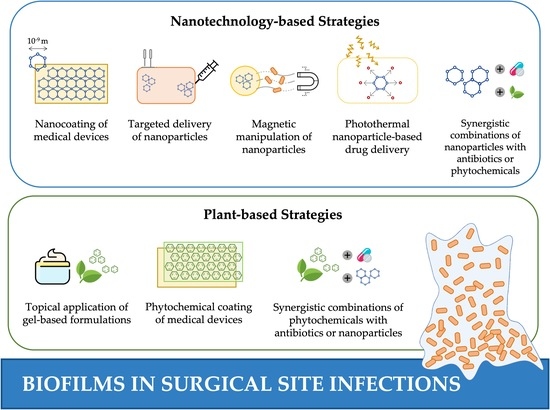
5. Novel Strategies to Control Biofilm-Associated SSIs
5.1. Nanotechnology-Based Strategies
5.2. Plant-Based Strategies
6. Implementation of Detection and Real-Time Monitoring Systems to Improve Biofilm Control Strategies
7. Concluding Remarks and Challenges
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andersen, B.M. Prevention of Postoperative Wound Infections. In Prevention and Control of Infections in Hospitals: Practice and Theory; Springer: Cham, Switzerland, 2019; pp. 377–437. ISBN 978-3-319-99921-0. [Google Scholar] [CrossRef]
- Young, P.Y.; Khadaroo, R.G. Surgical Site Infections. Surg. Clin. N. Am. 2014, 94, 1245–1264. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); National Healthcare Safety Network (NHSN). Available online: https://www.cdc.gov/nhsn/psc/ssi/index.html (accessed on 9 September 2021).
- Weiss, A.J.; Elixhauser, A.; Andrews, R.M. Characteristics of Operating Room Procedures in U.S. Hospitals, 2011; Statistical Brief, No. 170; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014. Available online: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb170-Operating-Room-Procedures-United-States-2011.pdf (accessed on 9 September 2021).
- McDermott, K.W.; Freeman, W.J.; Elixhauser, A. Overview of Operating Room Procedures During Inpatient Stays in U.S. Hospitals, 2014; Statistical Brief, No. 233; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. Available online: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb233-Operating-Room-Procedures-United-States-2014.pdf (accessed on 9 September 2021).
- European Centre for Disease Prevention and Control. Healthcare-Associated Infections: Surgical Site Infections; Annual Epidemiological Report for 2017; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-SSI.pdf (accessed on 9 September 2021).
- NIH Guide: Research on Microbial Biofilms. Available online: https://grants.nih.gov/grants/guide/pa-files/pa-03-047.html (accessed on 30 October 2021).
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L. Importance of Biofilm Formation in Surgical Infection. Br. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef] [Green Version]
- Wolcott, R.; Cutting, K.F.; Dowd, S.E. Surgical Site Infections: Biofilms, Dehiscence and Delayed Healing. Wounds UK 2008, 4, 108–113. [Google Scholar]
- Zabaglo, M.; Sharman, T. Postoperative Wound Infection; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Weber, S.; Herwaldt, L.A.; McNutt, L.-A.; Rhomberg, P.; Vaudaux, P.; Pfaller, M.A.; Perl, T.M. An Outbreak of Staphylococcus aureus in a Pediatric Cardiothoracic Surgery Unit. Infect. Control Hosp. Epidemiol. 2002, 23, 77–81. [Google Scholar] [CrossRef]
- Gyrska, P.; O’Dea, A.E. Postoperative Streptococcal Wound Infection: The Anatomy of an Epidemic. JAMA 1970, 213, 1189–1191. [Google Scholar] [CrossRef]
- Edmiston, C.E.; McBain, A.J.; Kiernan, M.; Leaper, D.J. A Narrative Review of Microbial Biofilm in Postoperative Surgical Site Infections: Clinical Presentation and Treatment. J. Wound Care 2016, 25, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Aga, E.; Keinan-Boker, L.; Eithan, A.; Mais, T.; Rabinovich, A.; Nassar, F. Surgical Site Infections after Abdominal Surgery: Incidence and Risk Factors. A Prospective Cohort Study. Infect. Dis. 2015, 47, 761–767. [Google Scholar] [CrossRef]
- Institute for Healthcare Improvement (IHI). Changes to Prevent Surgical Site Infection. Available online: http://www.ihi.org:80/resources/Pages/Changes/ChangestoPreventSurgicalSiteInfection.aspx (accessed on 15 September 2021).
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Salkind, A.R.; Rao, K.C. Antiobiotic Prophylaxis to Prevent Surgical Site Infections. Am. Fam. Physician 2011, 83, 585–590. [Google Scholar] [PubMed]
- Kathju, S.; Nistico, L.; Hall-Stoodley, L.; Post, J.C.; Ehrlich, G.D.; Stoodley, P. Chronic Surgical Site Infection Due to Suture-Associated Polymicrobial Biofilm. Surg. Infect. 2009, 10, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Renick, P.; Tang, L. Device-Related Infections. In Racing for the Surface: Pathogenesis of Implant Infection and Advanced Antimicrobial Strategies; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 171–188. ISBN 978-3-030-34475-7. [Google Scholar] [CrossRef]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, D.; Shivapriya, P.M.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A Review on Basic Biology of Bacterial Biofilm Infections and Their Treatments by Nanotechnology-Based Approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 243–259. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm Infections, Their Resilience to Therapy and Innovative Treatment Strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Baygar, T.; Sarac, N.; Ugur, A.; Karaca, I.R. Antimicrobial Characteristics and Biocompatibility of the Surgical Sutures Coated with Biosynthesized Silver Nanoparticles. Bioorgan. Chem. 2019, 86, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Ontong, J.C.; Wunnoo, S.; Paosen, S.; Munah, S.; Voravuthikunchai, S.P. Antibacterial-Coated Silk Surgical Sutures by Ex Situ Deposition of Silver Nanoparticles Synthesized with Eucalyptus camaldulensis Eradicates Infections. J. Microbiol. Methods 2020, 174, 105955. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Haj Bloukh, S.; Ibrahim, M.R.; Abu Sara, H. “Smart” Antimicrobial Nanocomplexes with Potential to Decrease Surgical Site Infections (SSI). Pharmaceutics 2020, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Syukri, D.M.; Nwabor, O.F.; Singh, S.; Voravuthikunchai, S.P. Antibacterial Functionalization of Nylon Monofilament Surgical Sutures through In Situ Deposition of Biogenic Silver Nanoparticles. Surf. Coat. Technol. 2021, 413, 127090. [Google Scholar] [CrossRef]
- Puca, V.; Traini, T.; Guarnieri, S.; Carradori, S.; Sisto, F.; Macchione, N.; Muraro, R.; Mincione, G.; Grande, R. The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection. Molecules 2019, 24, 2280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, J.; Zhu, R.; Lang, S.; Yan, H.; Liu, G.; Peng, B. Mussel-Inspired Immobilization of Zwitterionic Silver Nanoparticles toward Antibacterial Cotton Gauze for Promoting Wound Healing. Chem. Eng. J. 2021, 409, 128291. [Google Scholar] [CrossRef]
- Ständert, V.; Borcherding, K.; Bormann, N.; Schmidmaier, G.; Grunwald, I.; Wildemann, B. Antibiotic-Loaded Amphora-Shaped Pores on a Titanium Implant Surface Enhance Osteointegration and Prevent Infections. Bioact. Mater. 2021, 6, 2331–2345. [Google Scholar] [CrossRef]
- Surmeneva, M.; Lapanje, A.; Chudinova, E.; Ivanova, A.; Koptyug, A.; Loza, K.; Prymak, O.; Epple, M.; Ennen-Roth, F.; Ulbricht, M.; et al. Decreased Bacterial Colonization of Additively Manufactured Ti6Al4V Metallic Scaffolds with Immobilized Silver and Calcium Phosphate Nanoparticles. Appl. Surf. Sci. 2019, 480, 822–829. [Google Scholar] [CrossRef]
- Kurniawan, F.H.; Chinavinijkul, P.; Nasongkla, N. Hydrophobic and Antibacterial Bed Sheet Using ZnO Nanoparticles: A Large-Scale Technique. J. Drug Deliv. Sci. Technol. 2021, 62, 102339. [Google Scholar] [CrossRef]
- von Borowski, R.G.; Zimmer, K.R.; Leonardi, B.F.; Trentin, D.S.; Silva, R.C.; de Barros, M.P.; Macedo, A.J.; Gnoatto, S.C.B.; Gosmann, G.; Zimmer, A.R. Red Pepper Capsicum baccatum: Source of Antiadhesive and Antibiofilm Compounds against Nosocomial Bacteria. Ind. Crop. Prod. 2019, 127, 148–157. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Mariotti, C.E.; Conti, B.; Boccaccini, A.R. Electrophoretic Deposition of Ferulic Acid Loaded Bioactive Glass/Chitosan as Antibacterial and Bioactive Composite Coatings. Surf. Coat. Technol. 2021, 405, 126657. [Google Scholar] [CrossRef]
- Mir, M.; Permana, A.D.; Tekko, I.A.; McCarthy, H.O.; Ahmed, N.; Rehman, A.; Donnelly, R.F. Microneedle Liquid Injection System Assisted Delivery of Infection Responsive Nanoparticles: A Promising Approach for Enhanced Site-Specific Delivery of Carvacrol against Polymicrobial Biofilms-Infected Wounds. Int. J. Pharm. 2020, 587, 119643. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A. Enhancement in Site-Specific Delivery of Carvacrol against Methicillin Resistant Staphylococcus aureus Induced Skin Infections Using Enzyme Responsive Nanoparticles: A Proof of Concept Study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [Green Version]
- Okba, M.M.; Abdel Baki, P.M.; Abu-Elghait, M.; Shehabeldine, A.M.; El-Sherei, M.M.; Khaleel, A.E.; Salem, M.A. UPLC-ESI-MS/MS Profiling of the Underground Parts of Common Iris Species in Relation to Their Anti-Virulence Activities against Staphylococcus aureus. J. Ethnopharmacol. 2022, 282, 114658. [Google Scholar] [CrossRef]
- Đukanović, S.; Cvetković, S.; Lončarević, B.; Lješević, M.; Nikolić, B.; Simin, N.; Bekvalac, K.; Kekić, D.; Mitić-Ćulafić, D. Antistaphylococcal and Biofilm Inhibitory Activities of Frangula alnus Bark Ethyl-Acetate Extract. Ind. Crop. Prod. 2020, 158, 113013. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Ashour, R.M.; Okba, M.M.; Saber, F.R. Callistemon citrinus Bioactive Metabolites as New Inhibitors of Methicillin-Resistant Staphylococcus aureus Biofilm Formation. J. Ethnopharmacol. 2020, 254, 112669. [Google Scholar] [CrossRef]
- Alyousef, A.A.; Mabood Husain, F.; Arshad, M.; Rizwan Ahamad, S.; Shavez Khan, M.; Abul Qais, F.; Khan, A.; Alqasim, A.; Almutairi, N.; Ahmad, I.; et al. Myrtus communis and Its Bioactive Phytoconstituent, Linalool, Interferes with Quorum Sensing Regulated Virulence Functions and Biofilm of Uropathogenic Bacteria: In Vitro and In Silico Insights. J. King Saud Univ. Sci. 2021, 33, 101588. [Google Scholar] [CrossRef]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Narvi, S.S.; Agarwal, V. Exploring the Impact of Parthenolide as Anti-Quorum Sensing and Anti-Biofilm Agent against Pseudomonas aeruginosa. Life Sci. 2018, 199, 96–103. [Google Scholar] [CrossRef]
- Usmani, Y.; Ahmed, A.; Faizi, S.; Versiani, M.A.; Shamshad, S.; Khan, S.; Simjee, S.U. Antimicrobial and Biofilm Inhibiting Potential of an Amide Derivative [N-(2′, 4′-Dinitrophenyl)-3β-Hydroxyurs-12-En-28-Carbonamide] of Ursolic Acid by Modulating Membrane Potential and Quorum Sensing against Colistin Resistant Acinetobacter baumannii. Microb. Pathog. 2021, 157, 104997. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic Strategies against Bacterial Biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Feng, Y.; Coradi Tonon, C.; Ashraf, S.; Hasan, T. Photodynamic and Antibiotic Therapy in Combination against Bacterial Infections: Efficacy, Determinants, Mechanisms, and Future Perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113941. [Google Scholar] [CrossRef]
- Permana, A.D.; Anjani, Q.K.; Sartini; Utomo, E.; Volpe-Zanutto, F.; Paredes, A.J.; Evary, Y.M.; Mardikasari, S.A.; Pratama, M.R.; Tuany, I.N.; et al. Selective Delivery of Silver Nanoparticles for Improved Treatment of Biofilm Skin Infection Using Bacteria-Responsive Microparticles Loaded into Dissolving Microneedles. Mater. Sci. Eng. C 2021, 120, 111786. [Google Scholar] [CrossRef]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially Sensitive Nanoparticle-Based Dissolving Microneedles of Doxycycline for Enhanced Treatment of Bacterial Biofilm Skin Infection: A Proof of Concept Study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef]
- Gao, R.; van der Mei, H.C.; Ren, Y.; Chen, H.; Chen, G.; Busscher, H.J.; Peterson, B.W. Thermo-Resistance of ESKAPE-Panel Pathogens, Eradication and Growth Prevention of an Infectious Biofilm by Photothermal, Polydopamine-Nanoparticles In Vitro. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102324. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Feng, J.; Zhou, Y.; Wang, B. Synergistic Chemotherapy, Physiotherapy and Photothermal Therapy against Bacterial and Biofilms Infections through Construction of Chiral Glutamic Acid Functionalized Gold Nanobipyramids. Chem. Eng. J. 2020, 393, 124778. [Google Scholar] [CrossRef]
- Kirui, D.K.; Weber, G.; Talackine, J.; Millenbaugh, N.J. Targeted Laser Therapy Synergistically Enhances Efficacy of Antibiotics against Multi-Drug Resistant Staphylococcus aureus and Pseudomonas aeruginosa Biofilms. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102018. [Google Scholar] [CrossRef]
- Reifenrath, J.; Janßen, H.C.; Warwas, D.P.; Kietzmann, M.; Behrens, P.; Willbold, E.; Fedchenko, M.; Angrisani, N. Implant-Based Direction of Magnetic Nanoporous Silica Nanoparticles—Influence of Macrophage Depletion and Infection. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102289. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly(ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Shang, B.; Xu, M.; Zhi, Z.; Xi, Y.; Wang, Y.; Peng, B.; Li, P.; Deng, Z. Synthesis of Sandwich-Structured Silver@Polydopamine@Silver Shells with Enhanced Antibacterial Activities. J. Colloid Interface Sci. 2020, 558, 47–54. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is Combined Medication with Natural Medicine a Promising Therapy for Bacterial Biofilm Infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef]
- Aygül, A.; Şerbetçi, T. The Antibacterial and Antivirulent Potential of Hypericum lydium against Staphylococcus aureus: Inhibition of Growth, Biofilm Formation, and Hemolytic Activity. Eur. J. Integr. Med. 2020, 35, 101061. [Google Scholar] [CrossRef]
- Galvão, F.O.; Dantas, F.G.S.; Santos, C.R.L.; Marchioro, S.B.; Cardoso, C.A.L.; Wender, H.; Sangalli, A.; Almeida-Apolonio, A.A.; Oliveira, K.M.P. Cochlospermum regium (Schrank) Pilger Leaf Extract Inhibit Methicillin-Resistant Staphylococcus aureus Biofilm Formation. J. Ethnopharmacol. 2020, 261, 113167. [Google Scholar] [CrossRef]
- Ekom, S.E.; Tamokou, J.-D.-D.; Kuete, V. Methanol Extract from the Seeds of Persea americana Displays Antibacterial and Wound Healing Activities in Rat Model. J. Ethnopharmacol. 2022, 282, 114573. [Google Scholar] [CrossRef]
- Jain, A.; Parihar, D.K. Antibacterial, Biofilm Dispersal and Antibiofilm Potential of Alkaloids and Flavonoids of Curcuma. Biocatal. Agric. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Nadaf, N.H.; Parulekar, R.S.; Patil, R.S.; Gade, T.K.; Momin, A.A.; Waghmare, S.R.; Dhanavade, M.J.; Arvindekar, A.U.; Sonawane, K.D. Biofilm Inhibition Mechanism from Extract of Hymenocallis littoralis Leaves. J. Ethnopharmacol. 2018, 222, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Jardak, M.; Mnif, S.; Ben Ayed, R.; Rezgui, F.; Aifa, S. Chemical Composition, Antibiofilm Activities of Tunisian Spices Essential Oils and Combinatorial Effect against Staphylococcus epidermidis Biofilm. LWT 2021, 140, 110691. [Google Scholar] [CrossRef]
- Ferreira, G.R.S.; Brito, J.S.; Procópio, T.F.; Santos, N.D.L.; de Lima, B.J.R.C.; Coelho, L.C.B.B.; Navarro, D.M.A.F.; Paiva, P.M.G.; Soares, T.; de Moura, M.C.; et al. Antimicrobial Potential of Alpinia purpurata Lectin (ApuL): Growth Inhibitory Action, Synergistic Effects in Combination with Antibiotics, and Antibiofilm Activity. Microb. Pathog. 2018, 124, 152–162. [Google Scholar] [CrossRef]
- Lai, C.-S.; Ponnusamy, Y.; Lim, G.-L.; Ramanathan, S. Antibacterial, Antibiofilm and Antibiotic-Potentiating Effects of a Polyphenol-Rich Fraction of Dicranopteris linearis (Burm.f.) Underw. J. Herb. Med. 2021, 25, 100419. [Google Scholar] [CrossRef]
- Dias-Souza, M.V.; dos Santos, R.M.; Cerávolo, I.P.; Cosenza, G.; Ferreira Marçal, P.H.; Figueiredo, F.J.B. Euterpe oleracea Pulp Extract: Chemical Analyses, Antibiofilm Activity against Staphylococcus aureus, Cytotoxicity and Interference on the Activity of Antimicrobial Drugs. Microb. Pathog. 2018, 114, 29–35. [Google Scholar] [CrossRef]
- Neto, J.; Cabral, V.; Nogueira, L.F.; da Silva, C.; Sá, L.; da Silva, A.; da Silva, W.M.; Silva, J.; Marinho, E.S.; Cavalcanti, B.C.; et al. Anti-MRSA Activity of Curcumin in Planktonic Cells and Biofilms and Determination of Possible Action Mechanisms. Microb. Pathog. 2021, 155, 104892. [Google Scholar] [CrossRef]
- Deepika, M.; Thangam, R.; Sakthidhasan, P.; Arun, S.; Sivasubramanian, S.; Thirumurugan, R. Combined Effect of a Natural Flavonoid Rutin from Citrus sinensis and Conventional Antibiotic Gentamicin on Pseudomonas aeruginosa Biofilm Formation. Food Control 2018, 90, 282–294. [Google Scholar] [CrossRef]
- Durham, P.G.; Sidders, A.E.; Beam, J.E.; Kedziora, K.M.; Dayton, P.A.; Conlon, B.P.; Papadopoulou, V.; Rowe, S.E. Harnessing Ultrasound-Stimulated Phase Change Contrast Agents to Improve Antibiotic Efficacy against Methicillin-Resistant Staphylococcus aureus Biofilms. Biofilm 2021, 3, 100049. [Google Scholar] [CrossRef]
- da Silva, R.A.G.; Afonina, I.; Kline, K.A. Eradicating Biofilm Infections: An Update on Current and Prospective Approaches. Curr. Opin. Microbiol. 2021, 63, 117–125. [Google Scholar] [CrossRef]
- Le Gall, T.; Lemercier, G.; Chevreux, S.; Tücking, K.-S.; Ravel, J.; Thétiot, F.; Jonas, U.; Schönherr, H.; Montier, T. Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure–Activity Study on Clinical Bacterial Strains. ChemMedChem 2018, 13, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Collins, J.G.; Keene, F.R. Ruthenium Complexes as Antimicrobial Agents. Chem. Soc. Rev. 2015, 44, 2529–2542. [Google Scholar] [CrossRef] [Green Version]
- Frei, A.; Rubbiani, R.; Tubafard, S.; Blacque, O.; Anstaett, P.; Felgenträger, A.; Maisch, T.; Spiccia, L.; Gasser, G. Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2014, 57, 7280–7292. [Google Scholar] [CrossRef]
- Jiblaoui, A.; Leroy-Lhez, S.; Ouk, T.-S.; Grenier, K.; Sol, V. Novel Polycarboxylate Porphyrins: Synthesis, Characterization, Photophysical Properties and Preliminary Antimicrobial Study against Gram-Positive Bacteria. Bioorganic Med. Chem. Lett. 2015, 25, 355–362. [Google Scholar] [CrossRef]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Bayona, A.M.D.P.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design Features for Optimization of Tetrapyrrole Macrocycles as Antimicrobial and Anticancer Photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef] [Green Version]
- Juncker, R.; Lazazzera, B.; Billi, F. The Use of Functionalized Nanoparticles to Treat Staphylococcus aureus-based Surgical-Site Infections: A Systematic Review. J. Appl. Microbiol. 2021, 131, 2659–2668. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- John, A.; Shaji, A.; Velayudhannair, K.; Nidhin, M.; Krishnamoorthy, G. Anti-Bacterial and Biocompatibility Properties of Green Synthesized Silver Nanoparticles Using Parkia biglandulosa (Fabales:Fabaceae) Leaf Extract. Curr. Res. Green Sustain. Chem. 2021, 4, 100112. [Google Scholar] [CrossRef]
- Cruz, A.; Condinho, M.; Carvalho, B.; Arraiano, C.M.; Pobre, V.; Pinto, S.N. The Two Weapons against Bacterial Biofilms: Detection and Treatment. Antibiotics 2021, 10, 1482. [Google Scholar] [CrossRef]
- Thambirajoo, M.; Maarof, M.; Lokanathan, Y.; Katas, H.; Ghazalli, N.F.; Tabata, Y.; Fauzi, M.B. Potential of Nanoparticles Integrated with Antibacterial Properties in Preventing Biofilm and Antibiotic Resistance. Antibiotics 2021, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.M.; Tran, H.; Booth, M.A.; Fox, K.E.; Nguyen, T.H.; Tran, N.; Tran, P.A. Nanomaterials for Treating Bacterial Biofilms on Implantable Medical Devices. Nanomaterials 2020, 10, 2253. [Google Scholar] [CrossRef]
- do Nascimento, T.G.; da Silva, P.F.; Azevedo, L.F.; da Rocha, L.G.; de Moraes Porto, I.C.C.; Lima e Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; da Silva Fonseca, E.J.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef] [Green Version]
- Nie, L.; Chang, P.; Ji, C.; Zhang, F.; Zhou, Q.; Sun, M.; Sun, Y.; Politis, C.; Shavandi, A. Poly(Acrylic Acid) Capped Iron Oxide Nanoparticles via Ligand Exchange with Antibacterial Properties for Biofilm Applications. Colloids Surf. B Biointerfaces 2021, 197, 111385. [Google Scholar] [CrossRef]
- Porter, S.L.; Coulter, S.M.; Pentlavalli, S.; Thompson, T.P.; Laverty, G. Self-Assembling Diphenylalanine Peptide Nanotubes Selectively Eradicate Bacterial Biofilm Infection. Acta Biomater. 2018, 77, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Rozenbaum, R.T.; Andrén, O.C.J.; van der Mei, H.C.; Woudstra, W.; Busscher, H.J.; Malkoch, M.; Sharma, P.K. Penetration and Accumulation of Dendrons with Different Peripheral Composition in Pseudomonas aeruginosa Biofilms. Nano Lett. 2019, 19, 4327–4333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarena, A.S.; Gopal, S. Dendrimer a New Dimension in Targeting Biofilms. Mini-Rev. Med. Chem. 2013, 13, 1448–1461. [Google Scholar] [CrossRef]
- Ibaraki, H.; Kanazawa, T.; Chien, W.-Y.; Nakaminami, H.; Aoki, M.; Ozawa, K.; Kaneko, H.; Takashima, Y.; Noguchi, N.; Seta, Y. The Effects of Surface Properties of Liposomes on Their Activity against Pseudomonas aeruginosa PAO-1 Biofilm. J. Drug Deliv. Sci. Technol. 2020, 57, 101754. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, e3012462. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.F.; Kumar, G. Preliminary Phytochemical Screening and In Vitro Antibacterial Activity of Plumbago indica (Laal Chitrak) Root Extracts against Drug-Resistant Escherichia coli and Klebsiella pneumoniae. Open Agric. 2021, 6, 435–444. [Google Scholar] [CrossRef]
- Li, C.H.; Chen, X.; Landis, R.F.; Geng, Y.; Makabenta, J.M.; Lemnios, W.; Gupta, A.; Rotello, V.M. Phytochemical-Based Nanocomposites for the Treatment of Bacterial Biofilms. ACS Infect. Dis. 2019, 5, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Bennett, R.N.; Rosa, E.A.S. Understanding Antimicrobial Activities of Phytochemicals against Multidrug Resistant Bacteria and Biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Sakarikou, C.; Kostoglou, D.; Simões, M.; Giaouris, E. Exploitation of Plant Extracts and Phytochemicals against Resistant Salmonella spp. in Biofilms. Food Res. Int. 2020, 128, 108806. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; de Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-Biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, e566325. [Google Scholar] [CrossRef]
- Artini, M.; Patsilinakos, A.; Papa, R.; Božović, M.; Sabatino, M.; Garzoli, S.; Vrenna, G.; Tilotta, M.; Pepi, F.; Ragno, R.; et al. Antimicrobial and Antibiofilm Activity and Machine Learning Classification Analysis of Essential Oils from Different Mediterranean Plants against Pseudomonas aeruginosa. Molecules 2018, 23, 482. [Google Scholar] [CrossRef] [Green Version]
- Buetti-Dinh, A.; Galli, V.; Bellenberg, S.; Ilie, O.; Herold, M.; Christel, S.; Boretska, M.; Pivkin, I.V.; Wilmes, P.; Sand, W.; et al. Deep Neural Networks Outperform Human Expert’s Capacity in Characterizing Bioleaching Bacterial Biofilm Composition. Biotechnol. Rep. 2019, 22, e00321. [Google Scholar] [CrossRef]
- Ragi, S.; Rahman, M.H.; Duckworth, J.; Jawaharraj, K.; Chundi, P.; Gadhamshetty, V. Artificial Intelligence-Driven Image Analysis of Bacterial Cells and Biofilms. arXiv 2021, arXiv:2112.01577. [Google Scholar] [CrossRef]
- Kujundzic, E.; Cristina Fonseca, A.; Evans, E.A.; Peterson, M.; Greenberg, A.R.; Hernandez, M. Ultrasonic Monitoring of Early stage Biofilm Growth on Polymeric Surfaces. J. Microbiol. Methods 2007, 68, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, K.; Osgood, R.; Ren, D.; Pichichero, M.E.; Helguera, M. Ultrasound Imaging and Characterization of Biofilms Based on Wavelet De-Noised Radiofrequency Data. Ultrasound Med. Biol. 2014, 40, 583–595. [Google Scholar] [CrossRef]
- Lattwein, K.R.; Shekhar, H.; Kouijzer, J.J.P.; van Wamel, W.J.B.; Holland, C.K.; Kooiman, K. Sonobactericide: An Emerging Treatment Strategy for Bacterial Infections. Ultrasound Med. Biol. 2020, 46, 193–215. [Google Scholar] [CrossRef] [Green Version]
- LuTheryn, G.; Glynne-Jones, P.; Webb, J.S.; Carugo, D. Ultrasound-Mediated Therapies for the Treatment of Biofilms in Chronic Wounds: A Review of Present Knowledge. Microb. Biotechnol. 2020, 13, 613–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeor-Davidi, E.; Zverzhinetsky, M.; Krivitsky, V.; Patolsky, F. Real-Time Monitoring of Bacterial Biofilms Metabolic Activity by a Redox-Reactive Nanosensors Array. J. Nanobiotechnol. 2020, 18, 81. [Google Scholar] [CrossRef]
- Fang, K.; Park, O.-J.; Hong, S.H. Controlling Biofilms Using Synthetic Biology Approaches. Biotechnol. Adv. 2020, 40, 107518. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Rai, V.R. Effect of Small Chain N Acyl Homoserine Lactone Quorum Sensing Signals on Biofilms of Food-Borne Pathogens. J. Food Sci. Technol. 2016, 53, 3609–3614. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Yang, Z.; Pu, M.; Peti, W.; Wood, T.K. Engineering a Novel C-Di-GMP-Binding Protein for Biofilm Dispersal. Environ. Microbiol. 2011, 13, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, T.; Hassan, M.I. Modern Approaches in Synthetic Biology: Genome Editing, Quorum Sensing, and Microbiome Engineering. In Synthetic Biology: Omics Tools and Their Applications; Singh, S., Ed.; Springer: Singapore, 2018; pp. 189–205. ISBN 978-981-10-8693-9. [Google Scholar]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum Quenching: Role in Nature and Applied Developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Weiland-Bräuer, N.; Malek, I.; Schmitz, R.A. Metagenomic Quorum Quenching Enzymes Affect Biofilm Formation of Candida albicans and Staphylococcus epidermidis. PLoS ONE 2019, 14, e0211366. [Google Scholar] [CrossRef]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef] [Green Version]
- Pei, R.; Lamas-Samanamud, G.R. Inhibition of Biofilm Formation by T7 Bacteriophages Producing Quorum-Quenching Enzymes. Appl. Environ. Microbiol. 2014, 80, 5340–5348. [Google Scholar] [CrossRef] [Green Version]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Fang, K.; Jin, X.; Hong, S.H. Probiotic Escherichia coli Inhibits Biofilm Formation of Pathogenic E. coli via Extracellular Activity of DegP. Sci Rep. 2018, 8, 4939. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Fang, K.; Medina, D.; Wan, J.; Lee, J.-L.; Hong, S.H. The Probiotic, Leuconostoc mesenteroides, Inhibits Listeria monocytogenes Biofilm Formation. J. Food Saf. 2020, 40, e12750. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Koh, E.; Wong, A.; March, J.C.; Bentley, W.E.; Lee, Y.S.; Chang, M.W. Engineered Probiotic Escherichia coli Can Eliminate and Prevent Pseudomonas aeruginosa Gut Infection in Animal Models. Nat. Commun 2017, 8, 15028. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 27. [Google Scholar] [CrossRef]





| Type of Surgical Procedure | Bacterial Strain | Recommended Antibiotic(s) | Antibiotic Class(es) | Mode of Action |
|---|---|---|---|---|
| Cardiothoracic | S. aureus, coagulase-negative staphylococci | Cefazolin 1 | Cephalosporins | Disruption of peptidoglycan synthesis |
| Cefuroxime 2 | ||||
| Orthopedic | Vancomycin 3 | Aminoglycosides | Inhibition of protein synthesis | |
| Gastrointestinal | Enteric Gram-negative bacteria, anaerobes, enterococci | Cefoxitin 4 | Cephalosporins | Disruption of peptidoglycan synthesis |
| Cefotetan 5 | ||||
| Ampicillin 6/Sulbactam 7 | Beta-lactams | |||
| Cefazolin + Metronidazole 8 | Cephalosporins + Nitroimidazoles | Disruption of peptidoglycan synthesis + inhibition of protein synthesis and degradation of DNA | ||
| Gynecologic (vaginal, abdominal, or laparoscopic hysterectomy) | Enteric Gram-negative bacteria, group B streptococci, enterococci, anaerobes | Cefoxitin | Cephalosporins | Disruption of peptidoglycan synthesis |
| Cefotetan | ||||
| Cefazolin | ||||
| Ampicillin/Sulbactam | Beta-lactams | |||
| Vascular | S. aureus, coagulase-negative staphylococci, enteric Gram-negative bacilli | Cefazolin | Cephalosporins | Disruption of peptidoglycan synthesis |
| Vancomycin | Aminoglycosides | Inhibition of protein synthesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. https://doi.org/10.3390/antibiotics11010069
Hrynyshyn A, Simões M, Borges A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics. 2022; 11(1):69. https://doi.org/10.3390/antibiotics11010069
Chicago/Turabian StyleHrynyshyn, Andriy, Manuel Simões, and Anabela Borges. 2022. "Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies" Antibiotics 11, no. 1: 69. https://doi.org/10.3390/antibiotics11010069
APA StyleHrynyshyn, A., Simões, M., & Borges, A. (2022). Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics, 11(1), 69. https://doi.org/10.3390/antibiotics11010069