Essential Oils as a Good Weapon against Drug-Resistant Candida auris
Abstract
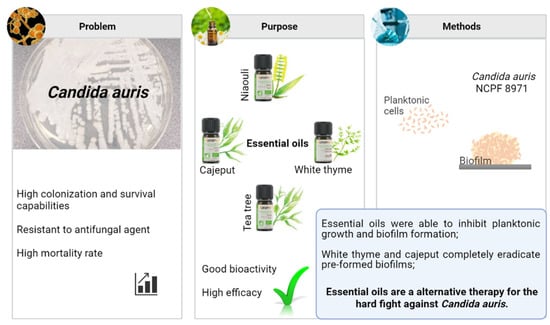
:1. Introduction
2. Results
2.1. Essential Oils Composition
2.2. Antifungal Susceptibility Testing
2.2.1. Fluconazole
2.2.2. Essential Oils
Planktonic Antimicrobial Susceptibilities
Biofilms Antimicrobial Susceptibilities
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Microorganisms and Culture Conditions in This Study
4.3. Antifungal Susceptibility Testing
4.3.1. Fluconazole
4.3.2. Essential Oils
Planktonic Antimicrobial Susceptibilities
Biofilm Antimicrobial Susceptibilities
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhary, A.; Voss, A.; Meis, J.F. Multidrug-Resistant Candida Auris: ‘New Kid on the Block’ in Hospital-Associated Infections? J. Hosp. Infect. 2016, 94, 209–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, N.A.; Gade, L.; Tsay, S.V.; Forsberg, K.; Greenko, J.A.; Southwick, K.L.; Barrett, P.M.; Kerins, J.L.; Lockhart, S.R.; Chiller, T.M.; et al. Multiple Introductions and Subsequent Transmission of Multidrug-Resistant Candida Auris in the USA: A Molecular Epidemiological Survey. Lancet Infect. Dis. 2018, 18, 1377–1384. [Google Scholar] [CrossRef]
- Aldejohann, A.M.; Wiese-Posselt, M.; Gastmeier, P.; Kurzai, O. Expert Recommendations for Prevention and Management of Candida Auris Transmission. Mycoses 2022, 65, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Browna, C.S. Candida Auris: A Review of the Literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef] [Green Version]
- Shaban, S.; Patel, M.; Ahmad, A. Improved Efficacy of Antifungal Drugs in Combination with Monoterpene Phenols against Candida Auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida Auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef] [Green Version]
- Rajkowska, K.; Nowicka-Krawczyk, P.; Kunicka-Styczynska, A. Effect of Clove and Thyme Essential Oils on Candida Biofilm Formation and the Oil Distribution in Yeast Cells. Molecules 2019, 24, 1954. [Google Scholar] [CrossRef] [Green Version]
- Palmeira-de-Oliveira, A.; Salgueiro, L.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Pina-Vaz, C.; Queiroz, J.; Rodrigues, A. Anti-Candida Activity of Essential Oils. Mini-Rev. Med. Chem. 2009, 9, 1292–1305. [Google Scholar] [CrossRef]
- Di Vito, M.; Mattarelli, P.; Modesto, M.; Girolamo, A.; Ballardini, M.; Tamburro, A.; Meledandri, M.; Mondello, F. In Vitro Activity of Tea Tree Oil Vaginal Suppositories against Candida Spp. and Probiotic Vaginal Microbiota. Phytother. Res. 2015, 29, 1628–1633. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid.-Based Complementary Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In Vitro Antifungal Activity of Cinnamomum Zeylanicum Bark and Leaf Essential Oils against Candida Albicans and Candida Auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911–8924. [Google Scholar] [CrossRef]
- Cosmétiques et Huiles Essentielles Bio Florame. Available online: https://fr.florame.com/?fbclid=IwAR34JDp1K64QMDTNYyauialnpoCUJlF3LBN9w85ZM8dYb4XCoL3OAnggVDQ (accessed on 2 November 2021).
- Antifungal Susceptibility Testing and Interpretation|Candida Auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 2 November 2021).
- Chatterjee, S.; Alampalli, S.V.; Nageshan, R.K.; Chettiar, S.T.; Joshi, S.; Tatu, U.S. Draft Genome of a Commonly Misdiagnosed Multidrug Resistant Pathogen Candida Auris. BMC Genom. 2015, 16, 686. [Google Scholar] [CrossRef] [Green Version]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The Effect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their Cyto/Genotoxic and Antioxidant Properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef] [Green Version]
- Mondello, F.; De Bernardis, F.; Girolamo, A.; Cassone, A.; Salvatore, G. In Vivo Activity of Terpinen-4-Ol, the Main Bioactive Component of Melaleuca Alternifolia Cheel (Tea Tree) Oil against Azole-Susceptible and -Resistant Human Pathogenic Candida Species. BMC Infect. Dis. 2006, 6, 158. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, R.; Fernandes, L.; Costa, R.; Cavaleiro, C.; Salgueiro, L.; Henriques, M.; Rodrigues, M.E. Comparing the Effect of Thymus Spp. Essential Oils on Candida Auris. Ind. Crops Prod. 2022, 178, 114667. [Google Scholar] [CrossRef]
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida Auris Forms High-Burden Biofilms in Skin Niche Conditions and on Porcine Skin. mSphere 2020, 5, e00910-19. [Google Scholar] [CrossRef] [Green Version]
- Asdadi, A.; Alilou, H.; Akssira, M.; Mina, L.; Hassani, I.; Chebli, B.; Moutaj, R.; Gonzặlez-Mas, C.; Amparo Blặzquez, M. Chemical Composition and Anticandidal Effect of Three Thymus Species Essential Oils from Southwest of Morocco against the Emerging Nosocomial Fluconazole-Resistant Strains. J. Biol. Agric. Healthc. 2014, 4, 16–26. [Google Scholar]
- Salgueiro, L.R.; Cavaleiro, C.; Gonçalves, M.J.; Proença Da Cunha, A. Antimicrobial Activity and Chemical Composition of the Essential Oil of Lippia Graveolens from Guatemala. Planta Med. 2003, 69, 80–83. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial Activity of Essential Oils from Australian Native Plants. Phytother. Res. 2005, 19, 643–646. [Google Scholar] [CrossRef]
- Keereedach, P.; Hrimpeng, K.; Boonbumrung, K. Antifungal Activity of Thai Cajuput Oil and Its Effect on Efflux-Pump Gene Expression in Fluconazole-Resistant Candida Albicans Clinical Isolates. Int. J. Microbiol. 2020, 2020, 5989206. [Google Scholar] [CrossRef]
- Oliva, B.; Piccirilli, E.; Ceddia, T.; Pontieri, E.; Aureli, P.; Ferrini, A.M. Antimycotic Activity of Melaleuca Alternifolia Essential Oil and Its Major Components. Lett. Appl. Microbiol. 2003, 37, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Zore, G.B.; Thakre, A.D.; Jadhav, S.; Karuppayil, S.M. Terpenoids Inhibit Candida Albicans Growth by Affecting Membrane Integrity and Arrest of Cell Cycle. Phytomedicine 2011, 18, 1181–1190. [Google Scholar] [CrossRef]
- Tampieri, M.P.; Galuppi, R.; MacChioni, F.; Carelle, M.S.; Falcioni, L.; Cioni, P.L.; Morelli, I. The Inhibition of Candida Albicans by Selected Essential Oils and Their Major Components. Mycopathologia 2005, 159, 339–345. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between Components of the Essential Oil of Melaleuca Alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Caetano, É.P.; de Lima, R.A.C.; Marques, F.J.D.F.; Castelo-Branco, D.D.S.C.M.; Melo, C.V.S.D.; Guedes, G.M.D.M.; Oliveira, J.S.D.; Camargo, Z.P.D.; Moreira, J.L.B.; et al. Terpinen-4-Ol, Tyrosol, and β-Lapachone as Potential Antifungals against Dimorphic Fungi. Braz. J. Microbiol. 2016, 47, 917. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Zhang, L.; An, P.; Qi, J.; Yu, X.; Lu, J.; Ren, X. Antifungal Mechanisms of α-Terpineol and Terpene-4-Alcohol as the Critical Components of Melaleuca alternifolia Oil in the Inhibition of Rot Disease Caused by Aspergillus ochraceus in Postharvest Grapes. J. Appl. Microbiol. 2019, 126, 1161–1174. [Google Scholar] [CrossRef]
- Muñoz, J.E.; Rossi, D.C.P.; Jabes, D.L.; Barbosa, D.A.; Cunha, F.F.M.; Nunes, L.R.; Arruda, D.C.; Taborda, C.P. In Vitro and In Vivo Inhibitory Activity of Limonene against Different Isolates of Candida spp. J. Fungi 2020, 6, 183. [Google Scholar] [CrossRef]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. Essential Oil to Inhibit the Growth of Food Spoiling Yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2017. [Google Scholar]
- Touati, I.; Ruiz, N.; Thomas, O.; Druzhinina, I.S.; Atanasova, L.; Tabbene, O.; Elkahoui, S.; Benzekri, R.; Bouslama, L.; Pouchus, Y.F.; et al. Hyporientalin A, an Anti-Candida Peptaibol from a Marine Trichoderma Orientale. World J. Microbiol. Biotechnol. 2018, 34, 98. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]


| Tea Tree Oil | Cajeput Oil | Niaouli Oil | White Thyme Oil | ||||
|---|---|---|---|---|---|---|---|
| Compounds | (%) | Compounds | (%) | Compounds | (%) | Compounds | (%) |
| Terpinen-4-ol | 42.20 | Cineole | 64.83 | p-Cymene +Limonene+1,8-cineole | 57.14 | Borneol | 31.86 |
| γ-terpinene | 21.38 | α-terpineol | 11.19 | α-pinene | 12.00 | α-terpineol | 15.95 |
| α-terpinene | 10.19 | Linalool | 3.21 | α-terpineol | 8.55 | Carvacrol | 8.63 |
| Terpinolene | 3.46 | Myrcene | 1.81 | Viridiflorol | 7.29 | Camphene | 7.26 |
| α-terpineol | 3.25 | α-pinene | 1.71 | β-pinene | 3.46 | trans-β-Caryophyllene | 6.62 |
| 1,8-cineole | 3.03 | γ-terpinene | 1.71 | β-Caryophyllene | 1.22 | Bornyl acetate + Thymol | 4.54 |
| α-pinene | 2.52 | Terpinolene | 1.31 | Ledene | 1.19 | α-pinene | 4.12 |
| p-Cymene | 1.49 | β-selinene | 1.28 | γ-terpinene | 1.04 | Linalool | 2.92 |
| Limonene | 1.08 | Sabinene | 1.23 | Terpinen-4-ol | 1.01 | p-Cymene | 2.14 |
| Aromadendrene | 1.00 | α-caryophyllene | 0.92 | Nerolidol | 0.73 | Terpinen-4-ol | 1.85 |
| Bicyclogermacrene | 0.85 | Terpinen-4-ol | 0.91 | Ledol | 0.73 | γ-terpinene | 1.83 |
| Ledene | 0.82 | α-selinene | 0.89 | β-myrcene | 0.56 | Camphor | 1.27 |
| δ-cadinene | 0.78 | Caryophyllene oxide | 0.89 | Alloaromadendrene | 0.38 | Limonene | 1.07 |
| Myrcene | 0.72 | β-eudesmol | 0.57 | Terpinolene | 0.34 | Thymol methyl ether | 0.82 |
| α-thujene | 0.71 | α-eudesmol | 0.55 | α-terpinene | 0.26 | β-pinene | 0.78 |
| β-phellandrene | 0.68 | α-terpinene | 0.54 | Linalool | 0.24 | 1,8-cineole | 0.73 |
| β-pinene | 0.66 | p-Cymene | 0.43 | Benzaldehyde | 0.22 | α-terpinene | 0.49 |
| Sabinene | 0.47 | Germacrene D | 0.33 | α-Caryophyllene | 0.20 | y-cadinene | 0.47 |
| α-phellandrene | 0.37 | α-phellandrene | 0.26 | β-selinene | 0.20 | δ-cadinene | 0.44 |
| Linalool | 0.36 | Borneol | 0.25 | y-cadinene | 0.18 | Terpinolene | 0.41 |
| Alloaromadendrene | 0.32 | Geranyl acetate | 0.25 | Camphene | 0.18 | Caryophyllene oxide | 0.36 |
| Globulol | 0.29 | α-thujene | 0.20 | δ-cadinene | 0.16 | Myrcene | 0.35 |
| α-gurgujene | 0.25 | Benzaldehyde | 0.16 | Isopulegol | 0.13 | Tricyclene | 0.33 |
| trans-β-Caryophyllene | 0.25 | 10-epi-gamma-eudesmol | 0.10 | α-phellandrene | 0.11 | α-thujene | 0.25 |
| Viridiflorol | 0.24 | α-Copaene | 0.06 | α-thujene | 0.11 | Ethanol | 0.17 |
| α-Copaene | 0.10 | Citronellol | 0.07 | ||||
| Alcool fenchylique | 0.07 | ||||||
| Borneol | 0.06 | ||||||
| Patchoulene | 0.06 | ||||||
| Methyl benzoate | 0.05 | ||||||
| trans-β-ocimene | 0.04 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, L.; Ribeiro, R.; Costa, R.; Henriques, M.; Rodrigues, M.E. Essential Oils as a Good Weapon against Drug-Resistant Candida auris. Antibiotics 2022, 11, 977. https://doi.org/10.3390/antibiotics11070977
Fernandes L, Ribeiro R, Costa R, Henriques M, Rodrigues ME. Essential Oils as a Good Weapon against Drug-Resistant Candida auris. Antibiotics. 2022; 11(7):977. https://doi.org/10.3390/antibiotics11070977
Chicago/Turabian StyleFernandes, Liliana, Rita Ribeiro, Raquel Costa, Mariana Henriques, and M. Elisa Rodrigues. 2022. "Essential Oils as a Good Weapon against Drug-Resistant Candida auris" Antibiotics 11, no. 7: 977. https://doi.org/10.3390/antibiotics11070977
APA StyleFernandes, L., Ribeiro, R., Costa, R., Henriques, M., & Rodrigues, M. E. (2022). Essential Oils as a Good Weapon against Drug-Resistant Candida auris. Antibiotics, 11(7), 977. https://doi.org/10.3390/antibiotics11070977