Staphylococcus Infection: Relapsing Atopic Dermatitis and Microbial Restoration
Abstract
:1. Introduction
2. Genetic Risk Factors and Epigenetic Regulators
2.1. Factors That Influence Staphylococcus Aureus Colonization of AD Skin
2.2. S. aureus Infection, Impairment of the Epidermal Barrier, and Potential Remodeling
3. The Initial Impact of SA Toxins and AD Recurrence
4. Restoration of Microbial Homeostasis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHR | Aryl hydrocarbon receptor |
| Blimp-1 | B lymphocyte-induced maturation protein-1 |
| Ccl2 | Chemokine ligand 2 |
| CoNS | Coagulase-negative staphylococci |
| E. coli | Escherichia coli |
| FLG | Filaggrin |
| FMT | Fecal matter transfer |
| Gamma | δ-Toxin |
| Hla | α−hemolysin |
| HlgAB | γ-hemolysin |
| HlgCB | γ-hemolysin |
| Jmjd3 | Jumonji domain-containing protein-3 |
| PSM | Phenol-soluble modulin |
| PVL | Panton-Valentine leukocidin |
| PVP | Polyvinyl-pyrrolidone |
| LOR | Loricrin |
| I3C | Indole-3-Carbinol |
| Irf4 | Interferon Regulatory Factor 4 |
| IVL | Involucrin |
| EDC | Epidermal differentiation complex |
| KLK | Kallikrein |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NRF2 | Nuclear factor E2-related factor 2 |
| Nos2 | Nitric oxide synthase 2 |
| SA | Staphylococcus aureus |
| SCFA | Short-chain fatty acids |
| SEA | Staphylococcal enterotoxins-A |
| SEB | Staphylococcal enterotoxins-B |
| SEC | Staphylococcal enterotoxins-C |
| SSRIs | Selective serotonin reuptake inhibitors |
| TL2 | Toll-like receptor 2 |
| Tnfa | Tumor necrosis factor alpha |
References
- Li, Y.; Su, J.; Luo, D.; Duan, Y.; Huang, Z.; He, M.; Tao, J.; Xiao, S.; Xiao, Y.; Chen, X.; et al. Processed Food and Atopic Dermatitis: A Pooled Analysis of Three Cross-Sectional Studies in Chinese Adults. Front. Nutr. 2021, 8, 754663. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.; Chu, S.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Nedley, N.; Delost, G.R. The Global, Regional, and National Burden of Atopic Dermatitis in 195 Countries and Territories: An Ecological Study from the Global Burden of Disease Study 2017. JAAD Int. 2021, 2, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadi, B.; Athari, S.M.; Abdollahi, M.; Vahedi, G.; Athari, S.S. Oral Administration of Acrylamide Worsens the Inflammatory Responses in the Airways of Asthmatic Mice Through Agitation of Oxidative Stress in the Lungs. Front. Immunol. 2020, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in Human Diet, Its Metabolism, Toxicity, Inactivation and the Associated European Union Legal Regulations in Food Industry. Crit. Rev. Food Sci. Nutr. 2019, 60, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Thammahong, A.; Kiatsurayanon, C.; Edwards, S.W.; Rerknimitr, P.; Chiewchengchol, D. The Clinical Significance of Fungi in Atopic Dermatitis. Int. J. Derm. 2020, 59, 926–935. [Google Scholar] [CrossRef]
- Girolomoni, G.; Busà, V.M. Flare Management in Atopic Dermatitis: From Definition to Treatment. Ther. Adv. Chronic Dis. 2022, 13, 20406223211066728. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M. The Eczema Area and Severity Index (EASI): Assessment of Reliability in Atopic Dermatitis. EASI Evaluator Group. Exp. Derm. 2001, 10, 11–18. [Google Scholar] [CrossRef]
- Bożek, A.; Reich, A. Assessment of Intra- and Inter-Rater Reliability of Three Methods for Measuring Atopic Dermatitis Severity: EASI, Objective SCORAD, and IGA. DRM 2017, 233, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Lull, C.; von Ahnen, J.A.; Gross, G.; Olsavszky, V.; Knitza, J.; Leipe, J.; Schmieder, A. German Mobile Apps for Patients With Psoriasis: Systematic Search and Evaluation. JMIR Mhealth Uhealth 2022, 10, e34017. [Google Scholar] [CrossRef]
- van Galen, L.S.; Xu, X.; Koh, M.J.A.; Thng, S.; Car, J. Eczema Apps Conformance with Clinical Guidelines: A Systematic Assessment of Functions, Tools and Content. Br. J. Dermatol. 2020, 182, 444–453. [Google Scholar] [CrossRef]
- Xu, X.; Griva, K.; Koh, M.; Lum, E.; Tan, W.S.; Thng, S.; Car, J. Creating a Smartphone App for Caregivers of Children With Atopic Dermatitis With Caregivers, Health Care Professionals, and Digital Health Experts: Participatory Co-Design. JMIR Mhealth Uhealth 2020, 8, e16898. [Google Scholar] [CrossRef]
- Ali, Z.; Chiriac, A.; Bjerre-Christensen, T.; Isberg, A.P.; Dahiya, P.; Manole, I.; Dutei, A.-M.; Deaconescu, I.; Serban, A.; Suru, A.; et al. Mild to Moderate Atopic Dermatitis Severity Can Be Reliably Assessed Using Smartphone-Photographs Taken by the Patient at Home: A Validation Study. Ski. Res. Technol. 2022, 28, 336–341. [Google Scholar] [CrossRef]
- Bang, C.H.; Yoon, J.W.; Ryu, J.Y.; Chun, J.H.; Han, J.H.; Lee, Y.B.; Lee, J.Y.; Park, Y.M.; Lee, S.J.; Lee, J.H. Automated Severity Scoring of Atopic Dermatitis Patients by a Deep Neural Network. Sci. Rep. 2021, 11, 6049. [Google Scholar] [CrossRef]
- Lyons, J.J.; Milner, J.D.; Stone, K.D. Atopic Dermatitis in Children: Clinical Features, Pathophysiology, and Treatment. Immunol. Allergy Clin. 2015, 35, 161–183. [Google Scholar] [CrossRef] [Green Version]
- Shi, B.; Leung, D.Y.M.; Taylor, P.A.; Li, H. Methicillin-Resistant Staphylococcus Aureus Colonization Is Associated with Decreased Skin Commensal Bacteria in Atopic Dermatitis. J. Investig. Derm. 2018, 138, 1668–1671. [Google Scholar] [CrossRef] [Green Version]
- Sugarman, J.L.; Hersh, A.L.; Okamura, T.; Howard, R.; Frieden, I.J. A Retrospective Review of Streptococcal Infections in Pediatric Atopic Dermatitis. Pediatr. Derm. 2011, 28, 230–234. [Google Scholar] [CrossRef]
- Möckel, M.; De La Cruz, N.C.; Rübsam, M.; Wirtz, L.; Tantcheva-Poor, I.; Malter, W.; Zinser, M.; Bieber, T.; Knebel-Mörsdorf, D. Herpes Simplex Virus 1 Can Bypass Impaired Epidermal Barriers upon Ex Vivo Infection of Skin from Atopic Dermatitis Patients. J. Virol. 2022, 96, e0086422. [Google Scholar] [CrossRef]
- Szczepańska, M.; Blicharz, L.; Nowaczyk, J.; Makowska, K.; Goldust, M.; Waśkiel-Burnat, A.; Czuwara, J.; Samochocki, Z.; Rudnicka, L. The Role of the Cutaneous Mycobiome in Atopic Dermatitis. J. Fungi 2022, 8, 1153. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic Dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Pellerin, L.; Henry, J.; Hsu, C.Y.; Balica, S.; Jean-Decoster, C.; Méchin, M.C.; Hansmann, B.; Rodriguez, E.; Weindinger, S.; Schmitt, A.M.; et al. Defects of Filaggrin-like Proteins in Both Lesional and Nonlesional Atopic Skin. J. Allergy Clin. Immunol. 2013, 131, 1094–1102. [Google Scholar] [CrossRef]
- Elmose, C.; Thomsen, S.F. Twin Studies of Atopic Dermatitis: Interpretations and Applications in the Filaggrin Era. J. Allergy 2015, 2015, 902359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef] [PubMed]
- Scharschmidt, T.C.; Man, M.Q.; Hatano, Y.; Crumrine, D.; Gunathilake, R.; Sundberg, J.P.; Silva, K.A.; Mauro, T.M.; Hupe, M.; Cho, S.; et al. Filaggrin Deficiency Confers a Paracellular Barrier Abnormality That Reduces Inflammatory Thresholds to Irritants and Haptens. J. Allergy Clin. Immunol. 2009, 124, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Jungersted, J.M.; Scheer, H.; Mempel, M.; Baurecht, H.; Cifuentes, L.; Høgh, J.K.; Hellgren, L.I.; Jemec, G.B.E.; Agner, T.; Weidinger, S. Stratum Corneum Lipids, Skin Barrier Function and Filaggrin Mutations in Patients with Atopic Eczema. Allergy 2010, 65, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Elias, M.S.; Bradley, M. Genetics in Atopic Dermatitis: Historical Perspective and Future Prospects. Acta Derm. Venereol. 2020, 100, adv00163. [Google Scholar] [CrossRef]
- Ju, Q.; Fimmel, S.; Hinz, N.; Stahlmann, R.; Xia, L.; Zouboulis, C.C. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Alters Sebaceous Gland Cell Differentiation in Vitro. Exp. Derm. 2011, 20, 320–325. [Google Scholar] [CrossRef]
- Rodríguez, E.; Baurecht, H.; Wahn, A.F.; Kretschmer, A.; Hotze, M.; Zeilinger, S.; Klopp, N.; Illig, T.; Schramm, K.; Prokisch, H.; et al. An Integrated Epigenetic and Transcriptomic Analysis Reveals Distinct Tissue-Specific Patterns of DNA Methylation Associated with Atopic Dermatitis. J. Investig. Derm. 2014, 134, 1873–1883. [Google Scholar] [CrossRef] [Green Version]
- Moltrasio, C.; Romagnuolo, M.; Marzano, A.V. Epigenetic Mechanisms of Epidermal Differentiation. Int. J. Mol. Sci. 2022, 23, 4874. [Google Scholar] [CrossRef]
- Mu, Z.; Zhang, J. The Role of Genetics, the Environment, and Epigenetics in Atopic Dermatitis. Adv. Exp. Med. Biol. 2020, 1253, 107–140. [Google Scholar] [CrossRef]
- Martin, M.J.; Estravís, M.; García-Sánchez, A.; Dávila, I.; Isidoro-García, M.; Sanz, C. Genetics and Epigenetics of Atopic Dermatitis: An Updated Systematic Review. Genes 2020, 11, 442. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, B.; Wang, C.; Wang, H.; Huang, P.; Pan, Y. MicroRNA-124 Alleviates Chronic Skin Inflammation in Atopic Eczema via Suppressing Innate Immune Responses in Keratinocytes. Cell Immunol. 2017, 319, 53–60. [Google Scholar] [CrossRef]
- Zeng, Y.-P.; Nguyen, G.H.; Jin, H.-Z. MicroRNA-143 Inhibits IL-13-Induced Dysregulation of the Epidermal Barrier-Related Proteins in Skin Keratinocytes via Targeting to IL-13Rα1. Mol. Cell. Biochem. 2016, 416, 63–70. [Google Scholar] [CrossRef]
- Rebane, A.; Runnel, T.; Aab, A.; Maslovskaja, J.; Rückert, B.; Zimmermann, M.; Plaas, M.; Kärner, J.; Treis, A.; Pihlap, M.; et al. MicroRNA-146a Alleviates Chronic Skin Inflammation in Atopic Dermatitis through Suppression of Innate Immune Responses in Keratinocytes. J. Allergy Clin. Immunol. 2014, 134, 836–847.e11. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yuan, W.; Yao, L.; Wang, S.; Jia, Z.; Wu, P.; Li, L.; Wei, P.; Wang, X.; et al. MicroRNA-155-5p Is a Key Regulator of Allergic Inflammation, Modulating the Epithelial Barrier by Targeting PKIα. Cell Death Dis. 2019, 10, 884. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhou, B.; Zhao, M.; Tang, J.; Lu, Q. Promoter Demethylation Contributes to TSLP Overexpression in Skin Lesions of Patients with Atopic Dermatitis. Clin. Exp. Derm. 2014, 39, 48–53. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D. Loricrin and Involucrin Expression Is Down-Regulated by Th2 Cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Takei, K.; Mitoma, C.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Galactomyces Fermentation Filtrate Prevents T Helper 2-Mediated Reduction of Filaggrin in an Aryl Hydrocarbon Receptor-Dependent Manner. Clin. Exp. Dermatol. 2015, 40, 786–793. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab Progressively Improves Systemic and Cutaneous Abnormalities in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Bierne, H.; Hamon, M.; Cossart, P. Epigenetics and Bacterial Infections. Cold Spring Harb. Perspect. Med. 2012, 2, a010272. [Google Scholar] [CrossRef]
- Pérez-Novo, C.A.; Zhang, Y.; Denil, S.; Trooskens, G.; De Meyer, T.; Van Criekinge, W.; Van Cauwenberge, P.; Zhang, L.; Bachert, C. Staphylococcal Enterotoxin B Influences the DNA Methylation Pattern in Nasal Polyp Tissue: A Preliminary Study. Allergy Asthma Clin. Immunol. 2013, 9, 48. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Foster, T.J. Cell Wall-Anchored Surface Proteins of Staphylococcus Aureus: Many Proteins, Multiple Functions. Curr. Top Microbiol. Immunol. 2017, 409, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Tauber, M.; Balica, S.; Hsu, C.-Y.; Jean-Decoster, C.; Lauze, C.; Redoules, D.; Viodé, C.; Schmitt, A.-M.; Serre, G.; Simon, M.; et al. Staphylococcus Aureus Density on Lesional and Nonlesional Skin Is Strongly Associated with Disease Severity in Atopic Dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1272–1274.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirofski, L.; Casadevall, A. The Meaning of Microbial Exposure, Infection, Colonisation, and Disease in Clinical Practice. Lancet Infect. Dis. 2002, 2, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; McLean, W.H.I. One Remarkable Molecule: Filaggrin. J. Investig. Derm. 2012, 132, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Benenson, S.; Zimhony, O.; Dahan, D.; Solomon, M.; Raveh, D.; Schlesinger, Y.; Yinnon, A.M. Atopic Dermatitis—A Risk Factor for Invasive Staphylococcus Aureus Infections: Two Cases and Review. Am. J. Med. 2005, 118, 1048–1051. [Google Scholar] [CrossRef]
- Goleva, E.; Berdyshev, E.; Leung, D.Y. Epithelial Barrier Repair and Prevention of Allergy. J. Clin. Investig. 2019, 129, 1463–1474. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus Aureus Extracellular Vesicles: A Story of Toxicity and the Stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef]
- Hong, S.-W.; Choi, E.-B.; Min, T.-K.; Kim, J.-H.; Kim, M.-H.; Jeon, S.G.; Lee, B.-J.; Gho, Y.S.; Jee, Y.-K.; Pyun, B.-Y.; et al. An Important Role of α-Hemolysin in Extracellular Vesicles on the Development of Atopic Dermatitis Induced by Staphylococcus Aureus. PLoS ONE 2014, 9, e100499. [Google Scholar] [CrossRef]
- Kim, M.-R.; Hong, S.-W.; Choi, E.-B.; Lee, W.-H.; Kim, Y.-S.; Jeon, S.G.; Jang, M.H.; Gho, Y.S.; Kim, Y.-K. Staphylococcus Aureus-Derived Extracellular Vesicles Induce Neutrophilic Pulmonary Inflammation via Both Th1 and Th17 Cell Responses. Allergy 2012, 67, 1271–1281. [Google Scholar] [CrossRef]
- White, J.R.; Dauros-Singorenko, P.; Hong, J.; Vanholsbeeck, F.; Phillips, A.; Swift, S. The Complex, Bidirectional Role of Extracellular Vesicles in Infection. Biochem. Soc. Trans. 2021, 49, 881–891. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Q.; Tang, J.; Xiong, L.; Li, L. Extracellular Vesicles: Emerging Therapeutics in Cutaneous Lesions. Int. J. Nanomed. 2021, 16, 6183–6202. [Google Scholar] [CrossRef]
- Wang, X.; Eagen, W.J.; Lee, J.C. Orchestration of Human Macrophage NLRP3 Inflammasome Activation by Staphylococcus Aureus Extracellular Vesicles. Proc. Natl. Acad. Sci. USA 2020, 117, 3174–3184. [Google Scholar] [CrossRef]
- Luz, B.S.R.D.; Nicolas, A.; Chabelskaya, S.; Rodovalho, V.D.R.; Le Loir, Y.; Azevedo, V.A.D.C.; Felden, B.; Guédon, E. Environmental Plasticity of the RNA Content of Staphylococcus Aureus Extracellular Vesicles. Front. Microbiol. 2021, 12, 634226. [Google Scholar] [CrossRef]
- Briaud, P.; Frey, A.; Marino, E.C.; Bastock, R.A.; Zielinski, R.E.; Wiemels, R.E.; Keogh, R.A.; Murphy, E.R.; Shaw, L.N.; Carroll, R.K. Temperature Influences the Composition and Cytotoxicity of Extracellular Vesicles in Staphylococcus Aureus. mSphere 2021, 6, e0067621. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Hanzelmann, D.; Härtner, T.; Peschel, A.; Götz, F. Skin-Specific Unsaturated Fatty Acids Boost the Staphylococcus Aureus Innate Immune Response. Infect. Immun. 2016, 84, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 143, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus Aureus in Atopic Dermatitis: Strain-Specific Cell Wall Proteins and Skin Immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Williams, M.R.; Nakatsuji, T.; Sanford, J.A.; Vrbanac, A.F.; Gallo, R.L. Staphylococcus Aureus Induces Increased Serine Protease Activity in Keratinocytes. J. Investig. Dermatol. 2017, 137, 377–384. [Google Scholar] [CrossRef]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.; Luster, A.D. T Cell Homing to Epithelial Barriers in Allergic Disease. Nat. Med. 2012, 18, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulme, J. Recent Advances in the Detection of Methicillin Resistant Staphylococcus Aureus (MRSA). BioChip J. 2017, 11, 89–100. [Google Scholar] [CrossRef]
- Kanchongkittiphon, W.; Gaffin, J.M.; Phipatanakul, W. Child with Atopic Dermatitis. Ann. Allergy Asthma Immunol. 2015, 114, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pondeljak, N.; Lugović-Mihić, L. Stress-Induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Kiecka, A.; Szczepanik, M. The Potential Action of SSRIs in the Treatment of Skin Diseases Including Atopic Dermatitis and Slow-Healing Wounds. Pharm. Rep. 2022, 74, 947–955. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Martinez-de-Oliveira, J.; Donders, G.G.G.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A. Anti-Candida Activity of Antidepressants Sertraline and Fluoxetine: Effect upon Pre-Formed Biofilms. Med. Microbiol. Immunol. 2018, 207, 195–200. [Google Scholar] [CrossRef]
- Kalaycı, S.; Demirci, S.; Sahin, F. Antimicrobial Properties of Various Psychotropic Drugs Against Broad Range Microorganisms. Curr. Psychopharmacol. 2014, 3, 195–202. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the Antimicrobial Action of Antidepressants on Gut Commensal Microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, X.; Li, G.; Gao, J.; Liang, Y. The Change of Gut Microbiota in MDD Patients under SSRIs Treatment. Sci. Rep. 2021, 11, 14918. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Tartar, D.M.; Bagood, M.D.; So, M.; Nguyen, A.V.; Gallegos, A.; Fregoso, D.; Serrano, J.; Nguyen, D.; Degovics, D.; et al. Topical Fluoxetine as a Novel Therapeutic That Improves Wound Healing in Diabetic Mice. Diabetes 2019, 68, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.F.; Mitsui, H.; Cardinale, I.; de Guzman, C.S.; Krueger, J.G.; et al. Progressive Activation of T(H)2/T(H)22 Cytokines and Selective Epidermal Proteins Characterizes Acute and Chronic Atopic Dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Bao, L.; Chan, L.S.; DiPietro, L.A.; Chen, L. Aberrant Wound Healing in an Epidermal Interleukin-4 Transgenic Mouse Model of Atopic Dermatitis. PLoS ONE 2016, 11, e0146451. [Google Scholar] [CrossRef]
- Stutte, S.; Quast, T.; Gerbitzki, N.; Savinko, T.; Novak, N.; Reifenberger, J.; Homey, B.; Kolanus, W.; Alenius, H.; Förster, I. Requirement of CCL17 for CCR7- and CXCR4-Dependent Migration of Cutaneous Dendritic Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 8736–8741. [Google Scholar] [CrossRef] [Green Version]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ishikawa, N.; Sugioka, T.; Koseki, H.; Kubosawa, H.; Kagawa, S.; Shimojo, N.; Ito, S.; Hattori, T. The Importance of Disinfection Therapy Using Povidone-Iodine Solution in Atopic Dermatitis. Dermatology 2002, 204 (Suppl. S1), 63–69. [Google Scholar] [CrossRef]
- Augustin, M.; Goepel, L.; Jacobi, A.; Bosse, B.; Mueller, S.; Hopp, M. Efficacy and Tolerability of Liposomal Polyvinylpyrrolidone-Iodine Hydrogel for the Localized Treatment of Chronic Infective, Inflammatory, Dermatoses: An Uncontrolled Pilot Study. Clin. Cosmet. Investig. Derm. 2017, 10, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- König, B.; Reimer, K.; Fleischer, W.; König, W. Effects of Betaisodona® on Parameters of Host Defense. DRM 1997, 195, 42–48. [Google Scholar] [CrossRef]
- Barakat, N.A.; Rasmy, S.A.; Hosny, A.E.-D.M.S.; Kashef, M.T. Effect of Povidone-Iodine and Propanol-Based Mecetronium Ethyl Sulphate on Antimicrobial Resistance and Virulence in Staphylococcus Aureus. Antimicrob. Resist. Infect. Control 2022, 11, 139. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone Iodine in Wound Healing: A Review of Current Concepts and Practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, M.J.; Westgate, S.J.; Mueller, S. Povidone-Iodine Ointment Demonstrates in Vitro Efficacy against Biofilm Formation. Int. Wound. J. 2017, 14, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hulme, J. Application of Nanomaterials in the Prevention, Detection, and Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA). Pharmaceutics 2022, 14, 805. [Google Scholar] [CrossRef] [PubMed]
- Giau, V.V.; An, S.S.A.; Hulme, J. Recent Advances in the Treatment of Pathogenic Infections Using Antibiotics and Nano-Drug Delivery Vehicles. Drug Des. Dev. Ther. 2019, 13, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieber, T. Atopic Dermatitis: An Expanding Therapeutic Pipeline for a Complex Disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Ito, Y.; Amagai, M. Controlling Skin Microbiome as a New Bacteriotherapy for Inflammatory Skin Diseases. Inflamm. Regen. 2022, 42, 26. [Google Scholar] [CrossRef]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the Human Skin Microbiota and Its Roles in Cutaneous Diseases. Microb. Cell Factories 2022, 21, 176. [Google Scholar] [CrossRef]
- Giau, V.V.; Lee, H.; An, S.S.A.; Hulme, J. Recent Advances in the Treatment of C. Difficile Using Biotherapeutic Agents. Infect. Drug Resist. 2019, 12, 1597–1615. [Google Scholar] [CrossRef] [Green Version]
- Trzeciak, M.; Sakowicz-Burkiewicz, M.; Wesserling, M.; Dobaczewska, D.; Gleń, J.; Nowicki, R.; Pawelczyk, T. Expression of Cornified Envelope Proteins in Skin and Its Relationship with Atopic Dermatitis Phenotype. Acta Derm. Venereol. 2017, 97, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- De Juan, A.; Segura, E. Modulation of Immune Responses by Nutritional Ligands of Aryl Hydrocarbon Receptor. Front. Immunol. 2021, 12, 1948. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, G.; Liu, X.; Shao, Y.; Gao, P.; Xin, C.; Cui, Z.; Zhao, X.; Xu, G. Serum Metabolomics Study and Eicosanoid Analysis of Childhood Atopic Dermatitis Based on Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2014, 13, 5715–5723. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial Control of Astrocytes in Response to Microbial Metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Schjødt, M.S.; Gürdeniz, G.; Chawes, B. The Metabolomics of Childhood Atopic Diseases: A Comprehensive Pathway-Specific Review. Metabolites 2020, 10, 511. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Arvola, T.; Salminen, S.J.; Isolauri, E. Aberrant Composition of Gut Microbiota of Allergic Infants: A Target of Bifidobacterial Therapy at Weaning? Gut 2002, 51, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and Its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Guzior, D.V.; Quinn, R.A. Review: Microbial Transformations of Human Bile Acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Yu, J.; Luo, Y.; Zhu, Z.; Zhou, Y.; Sun, L.; Gao, J.; Sun, J.; Wang, G.; Yao, X.; Li, W. A Tryptophan Metabolite of the Skin Microbiota Attenuates Inflammation in Patients with Atopic Dermatitis through the Aryl Hydrocarbon Receptor. J. Allergy Clin. Immunol. 2019, 143, 2108–2119.e12. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Pan, T.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Limosilactobacillus Reuteri Attenuates Atopic Dermatitis via Changes in Gut Bacteria and Indole Derivatives from Tryptophan Metabolism. Int. J. Mol. Sci. 2022, 23, 7735. [Google Scholar] [CrossRef]
- Sugiura, K. Unfolded Protein Response in Keratinocytes: Impact on Normal and Abnormal Keratinization. J. Dermatol. Sci. 2013, 69, 181–186. [Google Scholar] [CrossRef]
- Nakada, E.M.; Bhakta, N.R.; Korwin-Mihavics, B.R.; Kumar, A.; Chamberlain, N.; Bruno, S.R.; Chapman, D.G.; Hoffman, S.M.; Daphtary, N.; Aliyeva, M.; et al. Conjugated Bile Acids Attenuate Allergen-Induced Airway Inflammation and Hyperresponsiveness by Inhibiting UPR Transducers. JCI Insight 2019, 4, e98101. [Google Scholar] [CrossRef] [PubMed]
- Van Giau, V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.Y.; Hulme, J. Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; Cha, A.; Lee, H.; Yoon, H.; Yoon, T.; Cho, B.; Lee, S.; Park, Y. N-3 Polyunsaturated Fatty Acids and Atopy in Korean Preschoolers. Lipids 2007, 42, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut Microbiota Profile in Children Affected by Atopic Dermatitis and Evaluation of Intestinal Persistence of a Probiotic Mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, K.; Kim, W. Gut Microbiota Restoration through Fecal Microbiota Transplantation: A New Atopic Dermatitis Therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef]
- Mashiah, J.; Karady, T.; Fliss-Isakov, N.; Sprecher, E.; Slodownik, D.; Artzi, O.; Samuelov, L.; Ellenbogen, E.; Godneva, A.; Segal, E.; et al. Clinical Efficacy of Fecal Microbial Transplantation Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. Immun. Inflamm. Dis. 2022, 10, e570. [Google Scholar] [CrossRef]
- Graham, E.H.; Clarke, J.L.; Fernando, S.C.; Herr, J.R.; Adamowicz, M.S. The Application of the Skin Virome for Human Identification. Forensic Sci. Int. Genet. 2022, 57, 102662. [Google Scholar] [CrossRef]
- Mujtaba, M.G.; Johnson, H.M.; Parrish, J.M. Staphylococcal Enterotoxin Superantigens Induce Prophylactic Antiviral Activity Against Encephalomyocarditis Virus In Vivo and In Vitro. Viral. Immunol. 2021, 34, 392–400. [Google Scholar] [CrossRef]





| Infectious Species | Clinical Features | Immune Dysfunction | Microbiome | Reference |
|---|---|---|---|---|
| S. aureus and Methicillin-resistant Staphylococcus aureus (MRSA) | Weeping, honey-colored crusts and pustules, both interfollicular and follicular-based (folliculitis) Abscesses, fever, and lymphadenopathy | ↓Antimicrobial peptides ↑IL-13, IL-4 B-cell Ig class switching to IgE ↑type 2–related chemokines (CCL13, CCL17, CCL18, and CCL22) ↑Degradation of immunoglobulin G (IgG) | ↓ coagulase-negative Staphylococci (CoNS) (S. epidermidis, S. hominis, and S. lugdunensis) ↑ S. aureus | [14,15] |
| Beta-hemolytic streptococcal | Bright red erythema, thick-walled pustules, and heavy crusting | ↑Degradation of IgA, IgM, IgD, and IgE | ↑ S. aureus | [16] |
| Herpes simplex virus (HSV) molluscum contagiosum (MC), eczema vaccinia (EV), and eczema coxsackium (EC) | Superficial clusters of dome-shaped vesicles and/or small, round, punched-out erosions | ↑IL-13 and IL-4 ↓ IFN-γ and TNF-α | ↑ S. aureus | [17] |
| Malassezia globosa and Malassezia restricta nanovesicles | Pruritic monomorphous papules and/or pustules. Hypo- or hyper-pigmented non-inflammatory lesions | ↑ IgE↑ auto-reactive T cells induces autoreactivity to human proteins | ↓S. aureus | [18] |
| Date & Location | Trigger | Morphological Description | SCORAD | Treatment | Time to Resolution | Reference |
|---|---|---|---|---|---|---|
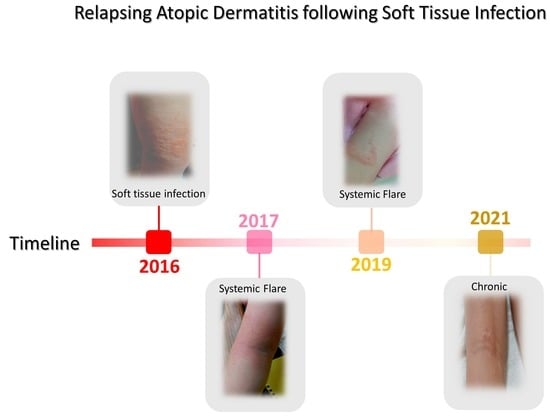
| 4 October 2016 A* | Fall and SA-infected abrasion | Marked erythema (deep or bright red), papulation; disease is widespread in extent | 56.87 | Permanganate (aq) cleansing high-strength oral antibiotics (amoxicillin) | 30 days | Figure 2A,B [63] |
| 26 October 2017 B* | Fall and abrasion | Perceptible erythema clearly perceptible induration/papulation | 43.7 | 1% Chloroxylenol bathing prior sleep | 16 days | Figure 3A–E |
| 27 December 2017 B* | Stress | Slight but definite erythema (pink), slight but definite induration | 19.45 | 1% Chloroxylenol bathing prior sleep | 12 days | N/A |
| 27 January 2018 A* | Fall and abrasion | Perceptible erythema induration/papulation | 26.9 | 1% Chloroxylenol bathing prior sleep | 16 days | N/A |
| 16 March 2019 A* | Heat and pressure | Perceptible scarring and Skin thickening (lichenification), itching | 27.8 | UV-B and exercise | 14 days | Figure 4A |
| 16 June 2021 C* | Heat and pressure | Perceptible scarring, persistent nodulation, itching Skin thickening (lichenification) | 21.8 | UV-B and exercise | 10 days | Figure 4B,C |
| 10 March 2022 C* | Foot wound | No inflammatory signs of local or systemic atopic dermatitis; nodulation and occasional itching | 7.4 | Topical application of Povidone | 5 days | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulme, J. Staphylococcus Infection: Relapsing Atopic Dermatitis and Microbial Restoration. Antibiotics 2023, 12, 222. https://doi.org/10.3390/antibiotics12020222
Hulme J. Staphylococcus Infection: Relapsing Atopic Dermatitis and Microbial Restoration. Antibiotics. 2023; 12(2):222. https://doi.org/10.3390/antibiotics12020222
Chicago/Turabian StyleHulme, John. 2023. "Staphylococcus Infection: Relapsing Atopic Dermatitis and Microbial Restoration" Antibiotics 12, no. 2: 222. https://doi.org/10.3390/antibiotics12020222
APA StyleHulme, J. (2023). Staphylococcus Infection: Relapsing Atopic Dermatitis and Microbial Restoration. Antibiotics, 12(2), 222. https://doi.org/10.3390/antibiotics12020222