Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Synthesis of AgNPs Coated with Drugs
2.3. Characterization of AgNPs-Coated Drugs
2.4. Bactericidal Assay
2.5. Cytopathogenicity Assay
3. Results
3.1. Characterization of Cephradine and Vildagliptin Coated Silver Nanoparticles
3.2. Cephradine and Vildagliptine Conjugated with AgNPs Exhibited Increased Bactericidal Effects against E. coli K1 and MRSA Compared with AgNPs and Drugs Alone
3.3. Cephradine and Vildagliptine Conjugated with AgNPs Exhibited Increased Bactericidal Effects against P. aeruginosa, K. pneumoniae, B. cereus, S. pyogenes Compared with the Drugs Alone
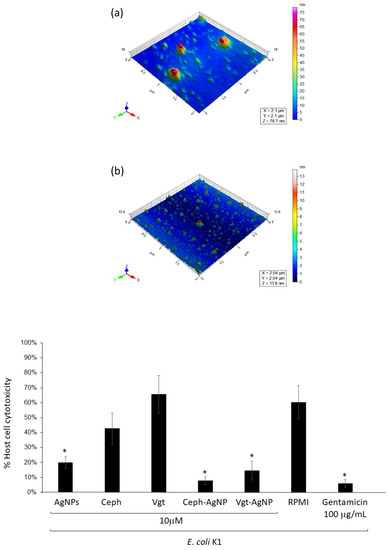
3.4. Silver Nanoparticle-Conjugated Drugs Inhibited E. coli K1-Mediated Host Cell Cytotoxicity
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garchitorena, A.; Sokolow, S.H.; Roche, B.; Ngonghala, C.N.; Jocque, M.; Lund, A.; Barry, M.; Mordecai, E.A.; Daily, G.C.; Jones, J.H.; et al. Disease ecology, health and the environment: A framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases. Philos. Trans. R. Soc. B 2017, 372, 20160128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, L.; Kong, X.; Sun, L. Application of nanodiagnostics in point-of-care tests for infectious diseases. Int. J. Nanomed. 2017, 12, 4789–4803. [Google Scholar] [CrossRef] [PubMed]
- Anuj, S.A.; Gajera, H.P.; Hirpara, D.G.; Golakiya, B.A. Bactericidal assessment of nano-silver on emerging and re-emerging human pathogens. J. Trace Elem. Med. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alanis, A.J. Resistance to antibiotics: Are we in the post-antibiotic era? Arch. Med. Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 2009, 62, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Pantosti, A.; Venditti, M. What is, M.R.SA? Eur. Respir. J. 2009, 34, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Granum, P.E.; Lund, T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1997, 157, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Z.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Grace, J.L.; Elliott, A.G.; Huang, J.X.; Schneider, E.K.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Quinn, J.F.; Velkov, T.; et al. Cationic acrylate oligomers comprising amino acid mimic moieties demonstrate improved antibacterial killing efficiency. J. Mater. Chem. B 2017, 5, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, Q.; Zhao, W.; Li, J.; Zhao, C. A self-defensive bilayer hydrogel coating with bacteria triggered switching from cell adhesion to antibacterial adhesion. Polym. Chem. 2017, 8, 5344–5353. [Google Scholar] [CrossRef]
- Yuan, H.; Yu, B.; Fan, L.H.; Wang, M.; Zhu, Y.; Ding, X.; Xu, F.J. Multiple types of hydroxyl-rich cationic derivatives of, P.G.MA for broad-spectrum antibacterial and antifouling coatings. Polym. Chem. 2016, 7, 5709–5718. [Google Scholar] [CrossRef]
- Grace, J.L.; Huang, J.X.; Cheah, S.E.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Quinn, J.F.; Velkov, T.; Whittaker, M.R. Antibacterial low molecular weight cationic polymers: Dissecting the contribution of hydrophobicity, chain length and charge to activity. RSC Adv. 2016, 6, 15469–15477. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Yang, X.; He, L.; Xu, X.; Liu, Y.; Liu, Y.; Gao, Y.; Huang, Q.; Liang, K.; Ding, C.; et al. Bio-inspired peptide decorated dendrimers for a robust antibacterial coating on hydroxyapatite. Polym. Chem. 2017, 8, 4264–4279. [Google Scholar] [CrossRef]
- Rabanal, F.; Grau-Campistany, A.; Vila-Farrés, X.; Gonzalez-Linares, J.; Borràs, M.; Vila, J.; Manresa, A.; Cajal, Y. A bioinspired peptide scaffold with high antibiotic activity and low in vivo toxicity. Sci. Rep. 2015, 5, 10558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshi, H.; Agarwal, S. Dendrons as active clicking tool for generating non-leaching antibacterial materials. Polym. Chem. 2016, 7, 5322–5330. [Google Scholar] [CrossRef] [Green Version]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Amador, P.; Prudêncio, C. β-Lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Barza, M.; Miao, P.V. Antimicrobial spectrum, pharmacology and therapeutic use of antibiotics. Part 3: Cephalosporins. Am. J. Health-Syst. Pharm. 1977, 34, 621–629. [Google Scholar]
- Ahrén, B.; Schweizer, A.; Dejager, S.; Villhauer, E.B.; Dunning, B.E. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes. Metab. 2011, 13, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Waghulde, M.; Naik, J. Comparative study of encapsulated vildagliptin microparticles produced by spray drying and solvent evaporation technique. Dry. Technol. 2017, 35, 1644–1654. [Google Scholar] [CrossRef]
- Nauck, M. Incretin therapies: Highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes. Metab. 2016, 18, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Shah, M.R.; Perveen, S.; Ahmed, S. Cephradine Coated Silver Nanoparticle their Drug Release Mechanism, and Antimicrobial Potential against Gram-Positive and Gram-Negative Bacterial Strains through AFM. J. Chem. Soc. Pakistan 2018, 40, 388–398. [Google Scholar]
- Anwar, A.; Khalid, S.; Perveen, S.; Ahmed, S.; Siddiqui, R. Synthesis of 4-(dimethylamino) pyridine propylthioacetate coated gold nanoparticles and their antibacterial and photophysical activity. J. Nanobiotechnol. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Osman, K.; Goldsworthy, G.J. Lysates of Locusta migratoria brain exhibit potent broad-spectrum antibacterial activity. J. Antimicrob. Chemother. 2008, 62, 634–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.M.; Siddiqui, R.; Ong, S.K.; Shah, M.R.; Anwar, A.; Heard, P.J.; Khan, N.A. Identification and characterization of antibacterial compound (s) of cockroaches (Periplaneta americana). Appl. Microbiol. Biotechnol. 2017, 101, 253–286. [Google Scholar] [CrossRef] [PubMed]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- De Lencastre, H.; Oliveira, D.; Tomasz, A. Antibiotic resistant Staphylococcus aureus: A paradigm of adaptive power. Curr. Opin. Microbiol. 2007, 10, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on, E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [PubMed]
- Shah, M.R.; Ali, S.; Ateeq, M.; Perveen, S.; Ahmed, S.; Bertino, M.F.; Ali, M. Morphological analysis of the antimicrobial action of silver and gold nanoparticles stabilized with ceftriaxone on Escherichia coli using atomic force microscopy. New J. Chem. 2014, 38, 5633–5640. [Google Scholar] [CrossRef]
- Rasheed, W.; Shah, M.R.; Perveen, S.; Ahmed, S.; Uzzaman, S. Revelation of susceptibility differences due to Hg (II) accumulation in Streptococcus pyogenes against, C.X.-AgNPs and Cefixime by atomic force microscopy. Ecotoxicol. Environ. Saf. 2018, 147, 9–16. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.B.; Raza Shah, M.; Khan, N.A. Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics 2018, 7, 100. https://doi.org/10.3390/antibiotics7040100
Masri A, Anwar A, Ahmed D, Siddiqui RB, Raza Shah M, Khan NA. Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics. 2018; 7(4):100. https://doi.org/10.3390/antibiotics7040100
Chicago/Turabian StyleMasri, Abdulkader, Ayaz Anwar, Dania Ahmed, Ruqaiyyah Bano Siddiqui, Muhammad Raza Shah, and Naveed Ahmed Khan. 2018. "Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin" Antibiotics 7, no. 4: 100. https://doi.org/10.3390/antibiotics7040100
APA StyleMasri, A., Anwar, A., Ahmed, D., Siddiqui, R. B., Raza Shah, M., & Khan, N. A. (2018). Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics, 7(4), 100. https://doi.org/10.3390/antibiotics7040100