Antifungal Activity in Compounds from the Australian Desert Plant Eremophila alternifolia with Potency Against Cryptococcus spp.
Abstract
:1. Introduction
2. Results
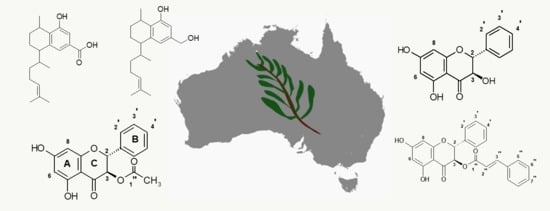
2.1. Identification of Compounds
2.2. Antifungal Screening Using the Disk Diffusion Assay
2.3. Antifungal Susceptibility using the Broth Microdilution Assay
3. Discussion
4. Materials and Methods
4.1. Collection and Description of Plant Materials
4.2. Solvents and Reagents
4.3. Equipment/Instruments Used
4.4. Extraction and Isolation
4.5. Chemical Synthesis
4.6. Fungal Strains
4.7. Antifungal Susceptibility Assays
4.7.1. Zone of Inhibition Assays
4.7.2. Broth Microdilution Assays
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, S.; Palombo, E.A. Characterisation of antibacterial Australian medicinal plant extracts by investigation of the mechanism of action and the effect of interfering substances. J. Basic Microbiol. 2005, 45, 363–370. [Google Scholar] [CrossRef]
- Cribb, A.B.; Cribb, J.W. Wild medicine in Australia; Fontana/Collins: Sydney, Australia, 1983. [Google Scholar]
- Ghisalberti, E.L. The ethnopharmacology and phytochemistry of Eremophila species (Myoporaceae). J. Ethnopharmacol. 1994, 44, 1–9. [Google Scholar] [CrossRef]
- Barr, A.; Chapman, J.; Smith, N.; Beveridge, M.; Knight, T.; Alexander, V.; Andrews, M. Traditional Bush Medicines: An Aboriginal Pharmacopoeia; Greenhouse Publications: Canberra, Australia, 1988. [Google Scholar]
- Chinnock, R.J. Eremophila and allied genera: a monograph of the plant family Myoporaceae; Rosenberg Publishing: Kenthurst, NSW, Australia, 2007. [Google Scholar]
- Singab, A.N.; Youssef, F.S.; Ashour, M.L.; Wink, M. The genus Eremophila (Scrophulariaceae): An ethnobotanical, biological and phytochemical review. J. Pharm. Pharmacol. 2013, 65, 1239–1279. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. The phytochemistry of the Myoporaceae. Phytochemistry 1993, 35, 7–33. [Google Scholar] [CrossRef]
- Liu, Q.; Harrington, D.; Kohen, J.L.; Vemulpad, S.; Jamie, J.F. Bactericidal and cyclooxygenase inhibitory diterpenes from Eremophila sturtii. Phytochemistry 2006, 67, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Ndi, C.P.; Semple, S.J.; Griesser, H.J.; Pyke, S.M.; Barton, M.D. Antimicrobial compounds from the Australian desert plant Eremophila neglecta. J. Nat. Prod. 2007, 70, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ndi, C.P.; Semple, S.J.; Griesser, H.J.; Pyke, S.M.; Barton, M.D. Antimicrobial compounds from Eremophila serrulata. Phytochemistry 2007, 68, 2684–2690. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Tucker, D.; Watson, K.; Jones, G.L. Identification of antibacterial constituents from the indigenous Australian medicinal plant Eremophila duttonii F. Muell.(Myoporaceae). J. Ethnopharmacol. 2007, 112, 386–393. [Google Scholar] [CrossRef]
- Barnes, E.C.; Kavanagh, A.M.; Ramu, S.; Blaskovich, M.A.; Cooper, M.A.; Davis, R.A. Antibacterial serrulatane diterpenes from the Australian native plant Eremophila microtheca. Phytochemistry 2013, 93, 162–169. [Google Scholar] [CrossRef]
- Mon, H.H.; Christo, S.N.; Ndi, C.P.; Jasieniak, M.; Rickard, H.; Hayball, J.D.; Griesser, H.J.; Semple, S.J. Serrulatane Diterpenoid from Eremophila neglecta Exhibits Bacterial Biofilm Dispersion and Inhibits Release of Pro-inflammatory Cytokines from Activated Macrophages. J. Nat. Prod. 2015, 78, 3031–3040. [Google Scholar] [CrossRef]
- Biva, I.J.; Ndi, C.P.; Griesser, H.J.; Semple, S.J. Antibacterial constituents of Eremophila alternifolia: An Australian aboriginal traditional medicinal plant. J. Ethnopharmacol. 2016, 182, 1–9. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Friends of The Waite Arboretum Inc. Available online: http://www.adelaide.edu.au/waite-historic/friends/arboretum/newsletters/fwa_nl_79_autumn_2014.pdf (accessed on 31 March 2019).
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef]
- Lai, C.C.; Tan, C.K.; Huang, Y.T.; Shao, P.L.; Hsueh, P.R. Current challenges in the management of invasive fungal infections. J Infect Chemother 2008, 14, 77–85. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. New Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef]
- Shourian, M.; Qureshi, S.T. Resistance and Tolerance to Cryptococcal Infection: An Intricate Balance That Controls the Development of Disease. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Di Mambro, T.; Guerriero, I.; Aurisicchio, L.; Magnani, M.; Marra, E. The Yin and Yang of Current Antifungal Therapeutic Strategies: How Can We Harness Our Natural Defenses? Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.D.; Ghisalberti, E.L.; Jefferies, P.R.; Raston, C.L.; White, A.H.; Hall, S.R. The chemistry of Eremophila SPP—VI*: Stereochemistry and crystal structure of dihydroxyserrulatic acid. Tetrahedron 1977, 33, 1475–1480. [Google Scholar] [CrossRef]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E.S. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Anakok, O.F.; Ndi, C.P.; Barton, M.D.; Griesser, H.J.; Semple, S.J. Antibacterial spectrum and cytotoxic activities of serrulatane compounds from the Australian medicinal plant Eremophila neglecta. J. Appl. Microbiol. 2012, 112, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, J.; Griesser, H.J.; Textor, M.; Landmann, R.; Khanna, N. Antimicrobial properties of 8-hydroxyserrulat-14-en-19-oic acid for treatment of implant-associated infections. Antimicrob. Agents Chemother. 2013, 57, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003, 95, 853–860. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2013, 114, 956–963. [Google Scholar] [CrossRef]
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef]
- Nett, J.E. Future directions for anti-biofilm therapeutics targeting Candida. Expert. Rev. Anti. Infect. Ther. 2014, 12, 375–382. [Google Scholar] [CrossRef]
- Barr, A. Traditional Bush Medicines: An Aboriginal Pharmacopoeia; Greenhouse Publications Pty Ltd.: Richmond Victoria, Australia, 1988. [Google Scholar]
- Pennacchio, M.; Alexander, E.; Ghisalberti, E.L.; Richmond, G.S. Cardioactive effects of Eremophila alternifolia extracts. J. Ethnopharmacol. 1995, 47, 91–95. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. Organic Chemistry 2001; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition; CLSI document M27-A3; Clinical and Laboratory Standards Institure: Wayne, NJ, USA, 2008.
- Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard CLSI document M38-A2; Clinical and Laboratory Standards Institure: Wayne, NJ, USA, 2008.
- Reference method for disk diffusion antifungal susceptibility testing of yeasts. Approved standard-second edition; CSLI document M44-A2; Clinical and Laboratory Standards institute: Wayne, NJ, USA, 2009.
- Method for antifungal disk diffusion susceptibility testing of filamentous fungi; proposed guideline, CSLI document M51-P; Clinical and Laboratory Standards institute: Wayne, NJ, USA, 2009.




| Fungal Strains | Incubation Time (hrs) | Zone of Inhibition* (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Standard Drugs | ||||||||
| 1 | 2 | 3 | 4 | 5 | CAS | NYS | AMB | ||
| Yeasts | |||||||||
| Candida albicans (ATCC 90028) | 24 | 7.7 ± 0.6 | 7.3 ± 0.6 | 7.1 ± 0.3 | 7.3 ± 0.3 | 7.3 ± 0.3 | 22.3 ± 0.6 | 26.5 ± 0.5 | 20.7 ± 0.6 |
| Candida tropicalis (ATCC 750) | 24 | NZ | NZ | NZ | NZ | NZ | 30.0 ± 1.7 | 25.7 ± 0.6 | 18.0 ± 1.0 |
| Candida parapsilosis (ATCC 22019) | 24 | NZ | NZ | NZ | NZ | NZ | 25.3 ± 1.2 | 17.3 ± 0.6 | 20.7 ± 0.6 |
| Candida glabrata (ATCC 90030) | 24 | NZ | NZ | NZ | NZ | NZ | 26.3 ± 0.6 | 25.6 ± 0.6 | 17.6 ± 0.6 |
| Candida krusei (ATCC 6258) | 24 | NZ | NZ | NZ | NZ | NZ | 25.6 ± 0.6 | 21.0 ± 1.0 | 18.3 ± 0.6 |
| Candida lusitaniae (ATCC 42720) | 24 | NZ | NZ | NZ | NZ | NZ | 24.0 ± 1.0 | 24.5 ± 1.3 | 21.5 ± 0.5 |
| Cryptococcus gattii (ATCC 32609) | 24 | 12.7 ± 0.6 | 11.6 ± 0.6 | 11.1 ± 0.3 | 11.3 ± 0.6 | 12.5 ± 0.5 | 14.0 ± 0.5 | 28.3 ± 0.6 | 29.0 ± 1.7 |
| Cryptococcus neoformans (ATCC 90113) | 24 | 11.1 ± 0.6 | 10.0 ± 1.0 | 10.8 ± 0.3 | 11.8 ± 0.3 | 11.7 ± 0.6 | 13.3 ± 0.6 | 24.0 ± 1.7 | 20.3 ± 1.2 |
| Moulds | |||||||||
| Aspergillus fumigatus (ATCC MYA 3626) | 24 | NZ | NZ | NZ | NZ | NZ | 31.7 ± 2.5 | 17.3 ± 1.1 | 20.0 ± 1.7 |
| Aspergillus niger (NMRC 14-41711737) | 24 | NZ | NZ | NZ | NZ | NZ | 26.3 ± 1.2 | 22.3 ± 1.2 | 22.7 ± 1.5 |
| Fungal Strains | Incubation Time (hrs) | MIC (µg/mL) (Modal MIC) * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Standard Drugs | ||||||||
| 1 | 2 | 3 | 4 | 5 | CAS | NYS | AMB | ||
| Yeasts | |||||||||
| Candida albicans (ATCC 90028) | 24 | 4–8 (4) | 128–256 (256) | 128–256 (256) | 64–128 (128) | 8–16 (16) | 0.06–0.12 (0.06) | 1–4 | 0.5–2 |
| Candida tropicalis (ATCC 750) | 24 | 16 | 64–128 (64) | 128–256 (256) | 64–128 (128) | 16–32 (32) | 0.06–0.12 (0.12) | 2–4 (2) | 0.5–1 (0.5) |
| Candida parapsilosis (ATCC 22019) | 48 | 8–16 (16) | 256 | 128–512 | 128–256 (256) | 64–128 (128) | 0.25–0.5 (0.5) | 2–4 (4) | 1–2 |
| Candida glabrata (ATCC 90030) | 48 | 16–32 (16) | 128–256 (256) | 256–512 (512) | 256–512 (256) | 32–64 (64) | 0.25–0.5 (0.25) | 1–2 (2) | 0.5–1 (0.5) |
| Candida krusei (ATCC 6258) | 48 | 4–8 (8) | 256 | 256–512 (512) | 128–256 (256) | 16–32 (32) | 2–4 (2) | 0.5–2 | 1–2 (1) |
| Candida lusitaniae (ATCC 42720) | 48 | 16–32 (16) | 128–256 (256) | 128–512 | 64–128 (128) | 64–128 (64) | 0.5–1 (0.5) | 2–4 (4) | 2–4 (2) |
| Cryptococcus gattii (ATCC 32609) | 48 | 4 | 128–256 (256) | 256–512 (512) | 128–256 (128) | 16–32 (32) | 8 | 2–4 (4) | 1–2 (1) |
| Cryptococcus neoformans (ATCC 90113) | 48 | 4 | 256 | 256–512 (512) | 128–256 (256) | 32–64 (64) | 8 | 2–8 | 0.5–2 (0.5) |
| Moulds | |||||||||
| Aspergillus fumigatus (ATCC MYA 3626) | 48 | ˃ 512 | ˃ 512 | ˃ 512 | ˃ 512 | ˃ 512 | 0.03–0.06 (0.03) | 4–8 (4) | 1–4 (1) |
| Aspergillus niger (NMRC 14-41711737) | 48 | 64 | 128 | ˃ 512 | ˃ 512 | ˃ 512 | 0.12 (0.06–0.25) | 4–8 (4) | 0.12–1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.A.; Biva, I.J.; Kidd, S.E.; Whittle, J.D.; Griesser, H.J.; Coad, B.R. Antifungal Activity in Compounds from the Australian Desert Plant Eremophila alternifolia with Potency Against Cryptococcus spp.. Antibiotics 2019, 8, 34. https://doi.org/10.3390/antibiotics8020034
Hossain MA, Biva IJ, Kidd SE, Whittle JD, Griesser HJ, Coad BR. Antifungal Activity in Compounds from the Australian Desert Plant Eremophila alternifolia with Potency Against Cryptococcus spp.. Antibiotics. 2019; 8(2):34. https://doi.org/10.3390/antibiotics8020034
Chicago/Turabian StyleHossain, Mohammed A., Israt J. Biva, Sarah E. Kidd, Jason D. Whittle, Hans J. Griesser, and Bryan R. Coad. 2019. "Antifungal Activity in Compounds from the Australian Desert Plant Eremophila alternifolia with Potency Against Cryptococcus spp." Antibiotics 8, no. 2: 34. https://doi.org/10.3390/antibiotics8020034
APA StyleHossain, M. A., Biva, I. J., Kidd, S. E., Whittle, J. D., Griesser, H. J., & Coad, B. R. (2019). Antifungal Activity in Compounds from the Australian Desert Plant Eremophila alternifolia with Potency Against Cryptococcus spp.. Antibiotics, 8(2), 34. https://doi.org/10.3390/antibiotics8020034