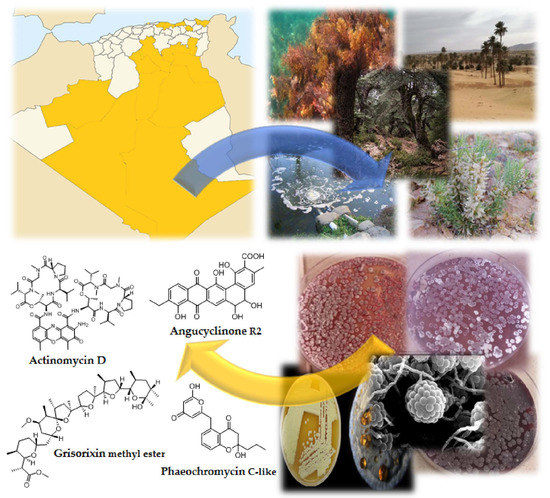
Actinobacteria Derived from Algerian Ecosystems as a Prominent Source of Antimicrobial Molecules
Abstract
:1. Introduction
2. Algerian Sampling Sites Providing Culturable Actinobacteria
3. Biodiversity of Rare and Novel Genera and Species of Actinobateria
4. Secondary Metabolites Derived from Algerian Actinobacteria
4.1. Antimicrobials
4.2. Other Activities
4.2.1. Tumor Cells Growth Inhibitors
4.2.2. Plant-Growth-Promoting Agents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 27 February 2017).
- Genilloud, O. Actinomycetes: Still a source of novel antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Antimicrobials from actinomycetes: Back to the future. Microbe 2007, 2, 125–131. [Google Scholar]
- Baltz, R.H. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 2008, 8, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, A.T. Actinobacteria of the extremobiosphere. In Extremophiles Handbook; Horikoshi, K., Ed.; Springer: Tokyo, Japan, 2011; pp. 1203–1240. [Google Scholar]
- Chater, K.F. Recent advances in understanding Streptomyces. F1000 Res. 2016, 5, 2795. [Google Scholar] [CrossRef] [PubMed]
- Van der Heul, H.U.; Bilyk, B.L.; McDowell, K.J.; Seipk, R.F.; Van Wezel, G.P. Regulation of antibiotic production in Actinobacteria: New perspectives from the post-genomicera. Nat. Prod. Rep. 2018, 35, 575–604. [Google Scholar] [CrossRef]
- Donadio, S.; Carrano, L.; Brandi, L.; Serina, S.; Soffientini, A.; Raimondi, E.; Montanini, N.; Sosio, M.; Gualerzi, C.O. Targets and assays for discovering novel antibacterial agents. J. Biotechnol. 2002, 3, 175–185. [Google Scholar] [CrossRef]
- Vrancken, K.; Anné, J. Secretory production of recombinant proteins by Streptomyces. Future Microbiol. 2009, 4, 181–188. [Google Scholar] [CrossRef]
- Zaho, X.Q.; Xu, X.N.; Che, L.Y. Production of enzymes from marine actinobacteria. In Marine Enzymes Biotechnology: Production and Industrial Applications, Part I—Production of Enzymes, 1st ed.; Kim, S.K., Toldrá, F., Eds.; Elsevier: Cambridge, MA, USA, 2016; Volume 78, pp. 137–151. [Google Scholar]
- Tang, B.; Xie, F.; Zhao, W.; Wang, J.; Dai, S.; Zheng, H.; Ding, X.; Cen, X.; Liu, H.; Yu, Y.; et al. A systematic study of the whole genome sequence of Amycolatopsis methanolica strain 239T provides an insight into its physiological andtaxonomic properties which correlate with its position in the genus. Synth. Syst. Biotechnol. 2016, 1, 169–186. [Google Scholar] [CrossRef]
- Tyurin, A.P.; Alferova, V.A.; Korshun, V.A. Chemical elicitors of antibiotic biosynthesis in Actinobacteria. Microorganisms 2018, 6, 52. [Google Scholar] [CrossRef]
- Labeda, D.P.; Goodfellow, M.; Brown, R.; Ward, A.C.; Lanoot, B.; Vanncanneyt, M.; Swings, J.; Kim, S.B.; Liu, Z.; Chun, J.; et al. Phylogenetic study of the species within the family Streptomycetaceae. Antonie Leeuwenhoek 2012, 101, 73–104. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.; Maciejewska, M.; Naômé, A.; Martinet, L.; Coppieters, W.; Karim, L.; Baurain, D.; Rigali, S. Isolation, characterization, and antibacterial activity of hard-to-culture actinobacteria from cave moonmilk deposits. Antibiotics 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Bredholt, H.; Fjærvik, E.; Johnsen, G.; Zotchev, S.B. Actinomycetes from sediments in the Trondheim Fjord, Norway: Diversity and biological activity. Mar. Drugs 2008, 6, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Okoro, C.K.; Brown, R.; Jones, A.L.; Andrews, B.A.; Asenjo, J.A.; Goodfellow, M.; Bull, A.T. Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie Leeuwenhoek 2009, 95, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Craveri, R.; Coronelli, C.; Pagani, H.; Sensi, P. Thermorubin, a new antibiotic from a thermoactinomycete. Clin. Med. 1964, 71, 511–521. [Google Scholar]
- Mohammadipanah, F.; Wink, J. Actinobacteria from arid and desert habitats: Diversity and biological activity. Front. Microbiol. 2016, 6, 1541–1550. [Google Scholar] [CrossRef]
- Li-Hua, H.; Qi-Ren, L.; Cheng-Lin, J. Diversity of soil actinomycetes in Japan and China. Appl. Environ. Microbiol. 1996, 62, 244–248. [Google Scholar]
- Lawrence, C. A method of isolating actinomycetes from scabby potato tissue and soil with minimal contamination. Can. J. Bot. 1956, 34, 44–47. [Google Scholar] [CrossRef]
- Sabaou, N.; Boudjella, H.; Bennadji, A.; Lamari, A.L.; Bennadji, H. Les sols des oasis du Sahara algérien sources d’actinomycètes rares producteurs d’antibiotiques. Secheresse 1998, 9, 45–53. [Google Scholar]
- Lamari, L.; Zitouni, A.; Dob, T.; Sabaou, N.; Germain, P.; Lefebvre, G.; Seguin, E.; Tillequin, F. New dithiolopyrrolone antibiotics from Saccharothrix sp. SA233. I. Taxonomy, production, isolation, and biological properties. J. Antibiot. 2002, 55, 696–701. [Google Scholar] [CrossRef]
- Zitouni, A.; Lamari, L.; Boudjella, H.; Badji, B.; Sabaou, N.; Gaouar, A.; Mathieu, F.; Lebrihi, A.; Labeda, D.P. Saccharothrix algeriensis sp. nov., isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2004, 54, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for fermentation medium optimization: An in-depth review. Front. Microbiol. 2016, 7, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Sircar, A.; Sridhar, P.; Das, P.K. Optimization of solid state medium for the production of clavulanic acid by Streptomyces clavuligerus. Process Biochem. 1998, 33, 283–289. [Google Scholar] [CrossRef]
- Gonzalez, R.; Islas, L.; Obregon, A.M.; Escalante, L.; Sanchez, S. Gentamicin formation in Micromonospora purpurea: Stimulatory effect of ammonium. J. Antibiot. 1995, 48, 479–483. [Google Scholar] [CrossRef]
- Haaland, P.D. Experimental Design in Biotechnology, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 1989; pp. 7–12. [Google Scholar]
- Keskin Gündoğdu, T.; Deniz, İ.; Çalışkan, G.; Şahin, E.S.; Azbar, N. Experimental design methods for bioengineering applications. Crit. Rev. Biotechnol. 2016, 36, 368–388. [Google Scholar] [CrossRef]
- Goupy, J. Plans D’expériences Pour Surfaces de Réponses, 1st ed.; Dunod: Paris, France, 1999; pp. 197–260. [Google Scholar]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual induction of new microbial secondary metabolites by fungal bacterial co-cultivation. Front. Microbiol. 2017, 8, 1284–1293. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Leeuwenhoek 2015, 108, 267–289. [Google Scholar] [CrossRef]
- Goudjal, Y.; Toumatia, O.; Yekkour, A.; Sabaou, N.; Mathieu, F.; Zitouni, A. Biocontrol of Rhizoctonia solani damping-off and promotion of tomato plant growth by endophytic actinomycetes isolated from native plants of Algerian Sahara. Microbiol. Res. 2014, 20, 59–65. [Google Scholar] [CrossRef]
- Zamoum, M.; Goudjal, Y.; Sabaou, N.; Barakate, M.; Mathieu, F.; Zitouni, A. Biocontrol capacities and plant growth-promoting traits of endophytic actinobacteria isolated from native plants of Algerian Sahara. J. Plant Dis. Prot. 2015, 122, 215–223. [Google Scholar] [CrossRef]
- Poulsen, M.; Oh, D.C.; Clardy, J.; Currie, C.R. Chemical analyses of wasp-associated Streptomyces bacteria reveal a prolific potential for natural products discovery. PLoS ONE 2011, 6, e16763. [Google Scholar] [CrossRef]
- Gomez-Escribano, J.P.; Alt, S.; Bibb, M.J. Next generation sequencing of Actinobacteria for the discovery of novel natural products. Mar. Drugs 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Duan, Y.; Huang, Y. The application of ribosome engineering to natural product discovery and yield improvement in Streptomyces. Antibiotics 2019, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Goudjal, Y.; Toumatia, O.; Sabaou, N.; Barakate, M.; Mathieu, F.; Zitouni, A. Endophytic actinomycetes from spontaneous plants of Algerian Sahara: Indole-3-acetic acid production and tomato plants growth promoting activity. World J. Microbiol. Biotechnol. 2013, 29, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Baoune, H.; Ould El Hadj-Khelil, A.; Pucci, G.; Sineli, P.; Loucif, L.; Polti, M.A. Petroleum degradation by endophytic Streptomyces spp. isolated from plants grown in contaminated soil of southern Algeria. Ecotoxicol. Environ. Safety 2018, 147, 602–609. [Google Scholar] [CrossRef]
- Belyagoubi, L.; Belyagoubi-Benhammou, N.; Jurado, V.; Dupont, J.; Lacoste, S.; Djebbah, F.; Ounadjela, F.Z.; Benaissa, S.; Habi, S.; Abdelouahid, D.E.; et al. Antimicrobial activities of culturable microorganisms (actinomycetes and fungi) isolated from Chaabe cave, Algeria. Int. J. Speleol. 2018, 47, 189–199. [Google Scholar] [CrossRef]
- Silini, S.; Ali-Khodja, H.; Boudemagh, A.; Terrouche, A.; Bouziane, M. Isolation and preliminary identification of actinomycetes isolated from a wastewater treatment plant and capable of growing on methyl ethyl ketone as a sole source of carbon and energy. Desalin. Water Treat. 2015, 57, 12108–12117. [Google Scholar] [CrossRef]
- Souagui, S.; Djoudi, W.; Boudries, H.; Béchet, M.; Leclère, V.; Kecha, M. Modeling and statistical optimization of culture conditions for improvement of antifungal compounds production by Streptomyces albidoflavus S19 strain of wastewater origin. Anti Infect. Agents. 2019, 17, 39–49. [Google Scholar] [CrossRef]
- Djinni, I.; Djoudi, W.; Souagui, S.; Rabia, F.; Rahmouni, S.; Mancini, I.; Kecha, M. Streptomyces thermoviolaceus SRC3 strain as a novel source of the antibiotic adjuvant streptazolin: A statistical approach toward the optimized production. J. Microbiol. Meth. 2018, 148, 161–168. [Google Scholar] [CrossRef]
- Meklat, A.; Bouras, N.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Schumann, P.; Sproer, C.; Klenk, H.P.; Sabaou, N. Actinopolyspora algeriensis sp. nov., a novel halophilic actinomycete isolated from a Saharan soil. Extremophiles 2012, 16, 771–776. [Google Scholar] [CrossRef]
- Meklat, A.; Bouras, N.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Schumann, P.; Sproer, C.; Klenk, H.P.; Sabaou, N. Actinopolyspora saharensis sp. nov., a novel halophilic actinomycete isolated from a Saharan soil of Algeria. Antonie Leeuwenhoek 2013, 103, 771–776. [Google Scholar] [CrossRef]
- Meklat, A.; Bouras, N.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Schumann, P.; Sproer, C.; Klenk, H.P.; Sabaou, N. Actinopolyspora righensis sp. nov., a novel halophilic actinomycete isolated from Saharan soil in Algeria. Antonie Leeuwenhoek 2013, 104, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Meklat, A.; Bouras, N.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Actinopolyspora mzabensis sp. nov., a halophilic actinomycete isolated from an Algerian Saharan soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 3787–3792. [Google Scholar] [CrossRef] [PubMed]
- Meklat, A.; Bouras, N.; Zitouni, A.; Sabaou, N.; Mathieu, F.; Schumann, P.; Sproer, C.; Klenk, H.P. Saccharopolyspora ghardaiensis sp. nov., an extremely halophilic actinomycete isolated from Algerian Saharan soil. J. Antibiot. 2014, 67, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Meklat, A.; Bouras, N.; Riba, A.; Zitouni, A.; Mathieu, F.; Rohde, M.; Schumann, P.; Sproer, C.; Klenk, H.P.; Sabaou, N. Streptomonospora algeriensis sp. nov., a halophilic actinomycete isolated from soil in Algeria. Antonie Leeuwenhoek 2014, 106, 287–292. [Google Scholar] [CrossRef]
- Meklat, A.; Bouras, N.; Mokrane, S.; Zitouni, A.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Bounagaea algeriensis gen. nov., sp. nov., an extremely halophilic actinobacterium isolated from a Saharan soil of Algeria. Antonie Leeuwenhoek 2015, 108, 473–482. [Google Scholar] [CrossRef]
- Souagui, Y.; Tritsch, D.; Grosdemange-Billiard, C.; Kecha, M. Optimization of antifungal production by an alkaliphilic and halotolerant actinomycete, Streptomyces sp. SY-BS5, using response surface methodology. J. Mycol. Med. 2015, 25, 108–115. [Google Scholar] [CrossRef]
- Sabaou, N.; Hacène, H.; Bennadji, A.; Bennadji, H.; Bounaga, N. Distribution quantitative et qualitative des actinomycètes dans les horizons des sols de surface et profonds d’une palmeraie algérienne. Can. J. Microbiol. 1992, 38, 1066–1073. [Google Scholar] [CrossRef]
- Djinni, I.; Defant, A.; Kecha, M.; Mancini, I. Antibacterial polyketides from the marine alga-derived endophitic Streptomyces sundarbansensis: A study on hydroxypyrone tautomerism. Mar. Drugs 2013, 11, 124–135. [Google Scholar] [CrossRef]
- Harir, M.; Bellahcene, M.; Baratto, M.C.; Pollini, S.; Rossolini, G.M.; Trabalzini, L.; Fatarella, E.; Pogni, R. Isolation and characterization of a novel tyrosinase produced by Sahara soil actinobacteria and immobilization on nylon nanofiber membranes. J. Biotechnol. 2018, 10, 54–64. [Google Scholar] [CrossRef]
- Hacène, H.; Sabaou, N.; Bounaga, N.; Lefebvre, G. Screening for non-polyenic antifungal antibiotics produced by rare Actinomycetales. Microbios 1994, 79, 81–85. [Google Scholar]
- Hacène, H.; Kebir, K.; Othmane, D.S.; Lefebvre, G. HM17, a new polyene antifungal antibiotic produced by a new strain of Spirillospora. J. Appl. Bacteriol. 1994, 77, 484–489. [Google Scholar] [CrossRef]
- Hacène, H.; Lefebvre, G. AH17, a new non-polyenic antifungal antibiotic produced by a strain of Spirillospora. Microbios 1995, 83, 199–205. [Google Scholar] [PubMed]
- Hacène, H.; Lefebvre, G. HP17, a new pigment-like antibiotic produced by a new strain of Spirillospora. J. Appl. Bacteriol. 1996, 80, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, N.; Ballif, M.; Zelleg, S.; Yousfi, N.; Ritter, C.; Friedel, U.; Amstutz, B.; Yala, D.; Boulahbal, F.; Guetarni, D.; et al. Mycobacterium algericum sp. nov., a novel rapidly growing species related to the Mycobacterium terrae complex and associated with goat lung lesions. Int. J. Syst. Evol. Microbiol. 2011, 61, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Boubetra, D.; Zitouni, A.; Bouras, N.; Mathieu, F.; Lebrihi, A.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Saccharothrix saharensis sp. nov., an actinomycete isolated from Algerian Saharan soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 3744–3749. [Google Scholar] [CrossRef]
- Boubetra, D.; Zitouni, A.; Bouras, N.; Mathieu, F.; Lebrihi, A.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Saccharothrix hoggarensis sp. nov., an actinomycete isolated from Saharan soil. J. Syst. Evol. Microbiol. 2013, 63549–63553. [Google Scholar]
- Saker, R.; Bouras, N.; Zitouni, A.; Ghoul, M.; Rohde, M.; Schumann, P.; Sproer, C.; Sabaou, N. Mzabimyces algeriensis gen. nov., sp. nov., a halophilic filamentous actinobacterium isolated from a Saharan soil, and proposal of Mzabimycetaceae fam. nov. Antonie Leeuwenhoek 2014, 106, 1021–1030. [Google Scholar] [CrossRef]
- Lai, H.; Jiang, Y.; Saker, R.; Chen, X.; Bouras, N.; Klenk, H.P.; Wei, X.; Jiang, Y.; Sabaou, N. Reclassification of Mzabimyces algeriensis Saker et al. 2015 as Halopolyspora algeriensis comb. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 2787–2790. [Google Scholar] [CrossRef]
- Aouiche, A.; Bouras, N.; Mokrane, S.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Actinokineospora mzabensis sp. nov., a novel actinomycete isolated from Saharan soil. Antonie Leeuwenhoek 2015, 107, 291–296. [Google Scholar] [CrossRef]
- Saker, R.; Bouras, N.; Meklat, A.; Zitouni, A.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Actinopolyspora biskrensis sp. nov., a novel halophilic actinomycete isolated from Northern Sahara. Curr. Microbiol. 2015, 70, 423–428. [Google Scholar] [CrossRef]
- Saker, R.; Bouras, N.; Meklat, A.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Prauserella isguenensis sp. nov., a halophilic actinomycete isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Bouras, N.; Meklat, A.; Zitouni, A.; Mathieu, F.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Nocardiopsis algeriensis sp. nov., an alkalitolerant actinomycete isolated from Saharan soil. Antonie Leeuwenhoek 2015, 107, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Boudjelal, F.; Zitouni, A.; Bouras, N.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Actinoalloteichus hoggarensis sp. nov., an actinomycete isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 2006–2010. [Google Scholar] [CrossRef] [PubMed]
- Boubetra, D.; Zitouni, A.; Bouras, N.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Saccharothrix tamanrassetensis sp. nov., an actinomycete isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2015, 65, 1316–1320. [Google Scholar] [CrossRef]
- Boubetra, D.; Bouras, N.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Streptosporangium algeriense sp. nov., an actinobacterium isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 1034–1038. [Google Scholar] [CrossRef]
- Lahoum, A.; Bouras, N.; Mathieu, F.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Actinomadura algeriensis sp. nov., an actinobacterium isolated from Saharan soil. Antonie Leeuwenhoek 2016, 109, 159–165. [Google Scholar] [CrossRef]
- Djouadi, L.N.; Levasseur, A.; Khalil, J.B.; Blanc-Taileur, C.; Asmar, S.; Ghiloubi, W.; Natèche, F.; Drancourt, M. Mycobacterium icosiumassiliensis sp. nov., a new member in the Mycobacterium terrae complex isolated from surface water in Algeria. Curr. Microbiol. 2016, 73, 255–264. [Google Scholar] [CrossRef]
- Lahoum, A.; Bouras, N.; Verheecke, C.; Mathieu, F.; Schumann, P.; Spröer, C.; Klenk, H.P.; Sabaou, N. Actinomadura adrarensis sp. nov., an actinobacterium isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2724–2729. [Google Scholar] [CrossRef]
- Bouznada, K.; Bouras, N.; Mokrane, S.; Chaabane Chaouch, F.; Zitouni, A.; Pötter, G.; Spröer, C.; Klenk, H.P.; Sabaou, N. Saccharothrix isguenensis sp. nov., an actinobacterium isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 4785–4790. [Google Scholar] [CrossRef]
- Bouznada, K.; Bouras, N.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Actinophytocola algeriensis sp. nov., an actinobacterium isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2760–2765. [Google Scholar] [CrossRef]
- Chaabane-Chaouch, F.; Bouras, N.; Mokrane, S.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Streptosporangium becharense sp. nov., an actinobacterium isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Chaabane-Chaouch, F.; Bouras, N.; Mokrane, S.; Zitouni, A.; Schumann, P.; Spröer, C.; Sabaou, N.; Klenk, H.P. Streptosporangium saharense sp. nov., an actinobacterium isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Bouznada, K.; Bouras, N.; Mokrane, S.; Chaabane-Chaouch, F.; Zitouni, A.; Pötter, G.; Spröer, C.; Klenk, H.P.; Sabaou, N. Saccharothrix ghardaiensis sp. nov., an actinobacterium isolated from Saharan soil. Antonie Leeuwenhoek 2017, 110, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chaabane-Chaouch, F.; Bouras, N.; Mokrane, S.; Bouznada, K.; Zitouni, A.; Pötter, G.; Spröer, C.; Klenk, H.P.; Sabaou, N. Planomonospora algeriensis sp. nov., an actinobacterium isolated from a Saharan soil of Algeria. Antonie Leeuwenhoek 2017, 110, 245–252. [Google Scholar] [CrossRef]
- Djaballah, C.E.; Kitouni, M.; Raoult, D.; Khelaifia, S. Streptomyces massilialgeriensis sp. nov., a new bacterial species isolated from an extremely saline soil collected from the dry lake of Ank el Djamel in Algeria. New Microbes New Infect. 2018, 21, 18–19. [Google Scholar] [CrossRef]
- Lamari, L.; Zitouni, A.; Dob, T.; Sabaou, N.; Lebrihi, A.; Germain, P.; Seguin, E.; Tillequin, F. New dithiolopyrrolone antibiotics from Saccharothrix sp. SA 233. II. Physicochemical properties and structure elucidation. J. Antibiot. 2002, 55, 702–706. [Google Scholar] [CrossRef]
- Merrouche, R.; Bouras, N.; Coppel, Y.; Mathieu, F.; Monje, M.C.; Sabaou, N.; Lebrihi, A. Dithiolopyrrolone antibiotic formation induced by adding valeric acid to the culture broth of Saccharothrix algeriensis. J. Nat. Prod. 2010, 73, 1164–1166. [Google Scholar] [CrossRef]
- Merrouche, R.; Bouras, N.; Coppel, Y.; Mathieu, F.; Sabaou, N.; Lebrihi, A. New dithiolopyrrolone antibiotics induced by adding sorbic acid to the culture medium of Saccharothrix algeriensis NRRL B-24137. FEMS Microbiol. Lett. 2011, 318, 41–46. [Google Scholar] [CrossRef]
- Merrouche, R.; Yekkour, A.; Coppel, Y.; Bouras, N.; Lamari, L.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Effective biosynthesis of benzoyl-pyrrothine dithiolopyrrolone antibiotic by cinnamic acid-precursor addition in culture of Saccharothrix algeriensis NRRL B-24137. Lett. Appl. Microbiol. 2019, 68, 165–172. [Google Scholar] [CrossRef]
- Zitouni, A.; Boudjella, H.; Mathieu, F.; Sabaou, N.; Lebrihi, A. Mutactimycin PR, a new anthracycline antibiotic from Saccharothrix sp. SA 103. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2004, 57, 367–372. [Google Scholar] [CrossRef]
- Zitouni, A.; Boudjella, H.; Lamari, L.; Badji, B.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Nocardiopsis and Saccharothrix genera in Saharan soils in Algeria: Isolation, biological activities and partial characterization of antibiotics. Res. Microbiol. 2005, 156, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Boudjella, H.; Bouti, K.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Taxonomy and chemical characterization of antibiotics of Streptosporangium Sg 10 isolated from a Saharan soil. Microbiol. Res. 2006, 161, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Boudjella, H.; Bouti, K.; Zitouni, A.; Mathieu, F.; Lebrihi, A.; Sabaou, N. Isolation and partial characterization of pigment-like antibiotics produced by a new strain of Streptosporangium isolated from an Algerian soil. J. Appl. Microbiol. 2007, 103, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Boudjella, H.; Zitouni, A.; Coppel, Y.; Mathieu, F.; Monje, M.C.; Sabaou, N.; Lebrihi, A. Antibiotic R2, a new angucyclinone compound from Streptosporangium sp. Sg3. J. Antibiot. 2010, 63, 709–711. [Google Scholar] [CrossRef]
- Badji, B.; Mostefaoui, A.; Sabaou, N.; Lebrihi, A.; Mathieu, F.; Seguin, E.; Tillequin, F. Isolation and partial characterization of antimicrobial compounds from a new strain Nonomuraea sp. NM94. J. Ind. Microbiol. Biotechnol. 2007, 34, 403–412. [Google Scholar] [CrossRef]
- Aouiche, A.; Sabaou, N.; Meklat, A.; Zitouni, A.; Bijani, C.; Mathieu, F.; Lebrihi, A. Saccharothrix sp. PAL54, a new chloramphenicol-producing strain isolated from a Saharan soil. World J. Microbiol. Biotechnol. 2012, 28, 943–951. [Google Scholar] [CrossRef]
- Djinni, I.; Defant, A.; Kecha, M.; Mancini, I. Metabolite profile of marine-derived endophytic Streptomyces sundarbansensis WR1L1S8 by liquid chromatography–mass spectrometry and evaluation of culture conditions on antibacterial activity and mycelial growth. J. Appl. Microbiol. 2013, 116, 39–50. [Google Scholar] [CrossRef]
- Boubetra, D.; Sabaou, N.; Zitouni, A.; Bijani, C.; Lebrihi, A.; Mathieu, F. Taxonomy and chemical characterization of new antibiotics produced by Saccharothrix SA198 isolated from a Saharan soil. Microbiol. Res. 2013, 168, 223–230. [Google Scholar] [CrossRef]
- Aouiche, A.; Bijani, C.; Zitouni, A.; Mathieu, F.; Sabaou, N. Antimicrobial activity of saquayamycins produced by Streptomyces spp. PAL114 isolated from a Saharan soil. J. Mycol. Med. 2014, 24, e17–e23. [Google Scholar] [CrossRef]
- Aouiche, A.; Meklat, A.; Bijani, C.; Zitouni, A.; Sabaou, N.; Mathieu, F. Production of vineomycin A1 and chaetoglobosin A by Streptomyces sp. PAL114. Ann. Microbiol. 2015, 65, 1351–1359. [Google Scholar] [CrossRef]
- Yekkour, A.; Meklat, A.; Bijani, C.; Toumatia, O.; Errakhi, R.; Lebrihi, A.; Mathieu, F.; Zitouni, A.; Sabaou, N. A novel hydroxamic acid-containing antibiotic produced by a Saharan soil-living Streptomyces strain. Lett. Appl. Microbiol. 2015, 60, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Driche, E.H.; Belghit, S.; Bijani, C.; Zitouni, A.; Sabaou, N.; Mathieu, F.; Badji, B. A new Streptomyces strain isolated from Saharan soil produces di-(2-ethylhexyl) phthalate, a metabolite active against methicillin-resistant Staphylococcus aureus. Ann. Microbiol. 2015, 65, 1341–1350. [Google Scholar] [CrossRef]
- Jalil, D.; Fakhre, N.A. Extraction, identification and determination of di-(2-ethylhexyl) phthalate (DEHP) plasticizer in some stored blood samples bags using different spectroscopic techniques. J. Pure Appl. Sci. 2016, 29, 155–170. [Google Scholar]
- Belghit, S.; Driche, E.H.; Bijani, C.; Zitouni, A.; Sabaou, N.; Badji, B.; Mathieu, F. Activity of 2,4-di-tert-butylphenol produced by a strain of Streptomyces mutabilis isolated from a Saharan soil against Candida albicans and other pathogenic fungi. J. Mycol. Med. 2016, 26, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Driche, E.H.; Sabaou, N.; Bijani, C.; Zitouni, A.; Pont, F.; Mathieu, F.; Badji, B. Streptomyces sp. AT37 isolated from a Saharan soil produces a furanone derivative active against multidrug-resistant Staphylococcus aureus. World J. Microbiol. Biotechnol. 2017, 33, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kesenheimer, C.; Groth, U. Total synthesis of (−)-8-O-methyltetrangomycin (MM 47755). Org. Lett. 2006, 8, 2507–2510. [Google Scholar] [CrossRef]
- Fotso, S.; Mahmud, T.; Zabriskie, T.M.; Santosa, D.A.; Sulastri; Proteau, P.J. Rearranged and unrearranged angucyclinones from Indonesian Streptomyces spp. J. Antibiot. 2008, 61, 449–456. [Google Scholar] [CrossRef]
- Hadj Rabia-Boukhalfa, Y.; Eveno, Y.; Karama, S.; Selama, O.; Lauga, B.; Duran, R.; Hacène, H.; Eparvier, V. Isolation, purification and chemical characterization of a new angucyclinone compound produced by a new halotolerant Nocardiopsis sp. HR-4 strain. World J. Microbiol. Biotechnol. 2017, 33, 126–135. [Google Scholar] [CrossRef]
- Khebizi, N.; Boudjella, H.; Bijani, C.; Bouras, N.; Klenk, H.P.; Pont, F.; Mathieu, F.; Sabaou, N. Oligomycins A and E, major bioactive secondary metabolites produced by Streptomyces sp. strain HG29 isolated from a Saharan soil. J. Mycol. Med. 2018, 28, 150–160. [Google Scholar] [CrossRef]
- Leulmi, N.; Sighel, D.; Defant, A.; Khenaka, K.; Boulahrouf, A.; Mancini, I. Nigericin and grisorixin methyl ester from the Algerian soil-living Streptomyces youssoufiensis SF10 strain: A computational study on their epimeric structures and evaluation of glioblastoma stem cells growth inhibition. Nat. Prod. Res. 2019, 33, 266–273. [Google Scholar] [CrossRef]
- Leulmi, N.; Sighel, D.; Defant, A.; Khenaka, K.; Boulahrouf, A.; Mancini, I. Enhanced production and quantitative evaluation of nigericin from the algerian soil-living Streptomyces youssoufiensis SF10 strain. Fermentation 2019, 5, 13. [Google Scholar] [CrossRef]
- Lahoum, A.; Sabaou, N.; Bijani, C.; Bouras, N.; Pont, F.; Snini, S.P.; Mathieu, F. Antimicrobial activities of novel bipyridine compounds produced by a new strain of Saccharothrix isolated from Saharan soil. Saudi Pharm. J. 2019, 27, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Liu, P.; Li, X.; Wang, Y.; Wang, S.; Hong, K.; Zhu, W. Cyclic bipyridine glycosides from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. Org. Lett. 2011, 13, 5948–5951. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Zhu, Y.; Mei, X.; Wang, Y.; Jia, H.; Zhang, C.; Zhu, W. Acyclic congeners from Actinoalloteichus cyanogriseus provide insights into cyclic bipyridine glycoside formation. Org. Lett. 2014, 16, 4264–4267. [Google Scholar] [CrossRef] [PubMed]
- Toumatia, O.; Yekkour, A.; Goudjal, Y.; Riba, A.; Coppel, Y.; Mathieu, F.; Sabaou, N.; Zitouni, A. Antifungal properties of an actinomycin D-producing strain, Streptomyces sp. IA1, isolated from a Saharan soil. J. Bas. Microbiol. 2015, 55, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Djinni, I.; Defant, A.; Djoudi, W.; Chaabane Chaouch, F.; Souagui, S.; Kecha, M.; Mancini, I. Modeling improved production of the chemotherapeutic polypeptide actinomycin D by a novel Streptomyces sp. strain from a Saharan soil. Heliyon 2019, 5, e01695. [Google Scholar] [CrossRef] [PubMed]
- Messis, A.; Bettache, A.; Brahami, A.; Kecha, M.; Benallaoua, S. Optimization of antifungal production from a novel strain Streptomyces sp. TKJ2 using response surface methodology. Med. Chem. Res. 2014, 23, 310–316. [Google Scholar] [CrossRef]
- Busi, S.; Pattnaik, S.S. Current status and applications of actinobacteria in the production of anticancerous compounds. In New and Future Developments in Microbial Biotechnology and Bioengineering, 1st ed.; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–153. [Google Scholar]
- Ravikumar, S.; Fredimoses, M.; Gnanadesigan, M. Anticancer property of sediment actinomycetes against MCF–7 and MDA–MB–231 cell lines. Asian Pac. J. Trop. Biomed. 2012, 2, 92–96. [Google Scholar] [CrossRef]
- Schmidt, C.; Shubert, N.A.; Brabetz, S.; Mack, N.; Schwalm, B.; Chan, J.A.; Selt, F.; Herold-Mend, C.; Witt, O.; Milde, T.; et al. Preclinical drug screen reveals topotecan, actinomycin D, and volasertib as potential new therapeutic candidates for ETMR brain tumor patients. Neuro Oncol. 2017, 19, 1607–1617. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.M.; Dong, T.T.; Feng, L.L.; Feng, B.; Zhao, H.C.; Fan, X.K.; Zheng, M.H. Suppression of colorectal cancer metastasis by nigericin through inhibition of epithelial-mesenchymal transition. World J. Gastroenterol. 2012, 18, 2640–2648. [Google Scholar] [CrossRef]
- Ameur, H.; Ghoul, M. Screening of actinomycetes producing antibacterial substances and indole acetic acid (IAA) and optimization of growth and IAA production conditions in Streptomyces sp. SF5. Int. J. Pharm. Biol. Arch. 2012, 3, 545–551. [Google Scholar]
- Merrouche, R.; Yekkour, A.; Lamari, L.; Zitouni, A.; Mathieu, F.; Sabaou, N. Efficiency of Saccharothrix algeriensis NRRL B-24137 and its produced antifungal dithiolopyrrolones compounds to suppress Fusarium oxysporum-induced wilt disease occurring in some cultivated crops. Arab. J. Sci. Eng. 2017, 42, 2321–2327. [Google Scholar] [CrossRef]
- Rugthaworn, P.; Dilokkunanant, U.; Sangchote, S.; Piadang, N.; Kitpreechavanich, V. A search and improvement of actinomycete strains for biological control of plant pathogens. Kasetsart J. 2007, 41, 248–254. [Google Scholar]
- Toumatia, O.; Compant, S.; Yekkour, A.; Goudjal, Y.; Sabaou, N.; Mathieu, F.; Sessitsch, A.; Zitouni, A. Biocontrol and plant growth promoting properties of Streptomyces mutabilis strain IA1 isolated from a Saharan soil on wheat seedlings and visualization of its niches of colonization. S. Afr. J. Bot. 2016, 105, 234–239. [Google Scholar] [CrossRef]







| Microorganism | Ecological Niches and Climate | References |
|---|---|---|
| Saccharothrix algeriensis sp. nov. | Saharan Palm grove, Adrar; Saharan climate | [24] |
| Mycobacterium algericum sp. nov. | Goat-lung lesion, Souk El Tenine slaughterhouse, Bejaia; Mediterranean climate | [59] |
| Actinopolyspora algeriensis sp. nov. | Saharan salin soil, Ouargla; Saharan climate | [44] |
| Actinopolyspora saharensis sp. nov. | Saharan soil, El Oued; Saharan climate | [45] |
| Actinopolyspora righensis sp. nov. | Saharan soil, El Oued; Saharan climate | [46] |
| Actinopolyspora mzabensis sp. nov. | Saharan soil, Ghardaia; Saharan climate | [47] |
| Saccharothrix saharensis sp. nov. | Saharan palm grove, Adrar; Saharan climate | [60] |
| Saccharothrix hoggarensis sp. nov. | Saharan soil, Hoggar–Tamenrasset; Saharan climate | [61] |
| Saccharopolyspora ghardaiensis sp. nov. | Saharan soil, Ghardaia; Saharan climate | [48] |
| Halopolyspora algeriensis comb. nov. | Saharan soil, Mzab region, Ghardaia; Saharan climate | [62,63] |
| Streptomonospora algeriensis sp. nov. | Soil sample, Djelfa; semiarid climate | [49] |
| Actinokineospora mzabensis sp. nov. | Saharan soil, Beni izguen region, Ghardaia; Saharan climate | [64] |
| Bounagaea algeriensis gen. nov., sp. nov. | Saharan soil, El-Goléa, Ghardaia; Saharan climate | [50] |
| Actinopolyspora biskrensis sp. nov. | Saharan soil, Biskra; Saharan climate | [65] |
| Prauserella isguenensis sp. nov. | Saharan soil, Beni izguen region, Ghardaia; Saharan climate | [66] |
| Nocardiopsis algeriensis sp. nov. | Saharan soil, Adrar; Saharan climate | [67] |
| Actinoalloteichus hoggarensis sp. nov. | Saharan soil, Hoggar region, Tamanrasset; Saharan climate | [68] |
| Saccharothrix tamanrassetensis sp. nov. | Saharan soil, Hoggar region, Tamanrasset; Saharan climate | [69] |
| Streptosporangium algeriense sp. nov. | Saharan soil, palm grove in Adrar; Saharan climate | [70] |
| Actinomadura algeriensis sp. nov. | Saharan soil, Hoggar region, Tamanrasset; Saharan climate | [71] |
| Mycobacterium icosiumassiliensis sp. nov. | Water lake surface, Algiers; Mediterranean climate | [72] |
| Actinomadura adrarensis sp. nov. | Saharan soil, Adrar; Saharan climate | [73] |
| Saccharothrix isguenensis sp. nov. | Saharan soil, Mzab region, Ghardaia; Saharan climate | [74] |
| Actinophytocola algeriensis sp. nov. | Saharan soil, Mzab region, Ghardaia; Saharan climate | [75] |
| Streptosporangium becharense sp. nov. | Saharan soil, Beni Abbes region, Bechar; Saharan climate | [76] |
| Streptosporangium saharense sp. nov. | Saharan soil, Mzab region, Ghardaia; Saharan climate | [77] |
| Saccharothrix ghardaiensis sp. nov. | Saharan soil, Mzab region, Ghardaia; Saharan climate | [78] |
| Planomonospora algeriensis sp. nov. | Saharan soil, Beni Abbas, Bechar; Saharan climate | [79] |
| Streptomyces massilialgeriensis sp. nov. | Saline soil, dry lake, Oum el Bouaghi; semiarid climate | [80] |
| Compound | Bioactivity | Producer Strain | Source | Reference |
|---|---|---|---|---|
| 3-Methyl-2-butenoylpyrrothine (1), Tigloylpyrrothine (2), n-Butyropyrrothine (3), iso-Butyropyrrothine (4), Thiolutin (5) | antibacterial, antifungal | Saccharothrix sp. SA 233 | Saharian palm grove soil (Adrar) | [24,81] |
| Valerylpyrrothine (6), Isovalerylpyrrothine (7), Formylpyrrothine (8), Aureothricin (9) | antibacterial and antifungal | Saccharothrix algeriensis NRRL B-24137, fully sequenced strain | Saharan soil | [82] |
| Crotonyl-pyrrothine (10), Sorbyl-pyrrothine (11), 2-Hexenyl-pyrrothine (12), 2-Methyl-3-pentenyl-pyrrothine (13) | antibacterial and antifungal | Saccharothrix algeriensis NRRL B-24137 | Saharan soil | [83] |
| Benzoyl-pyrrothine dithiolopyrrolone (14) | antibacterial, antifungal | Saccharothrix algeriensis NRRL B-24137 | palm grove soil (Southern Algeria) | [84] |
| Mutactimycin PR (15), Mutactimycin C (16) | moderate anti-Gram-positive bacteria | Saccharothrix sp. SA 103 | Saharan soil sample (Tamanrasset, South Algeria) | [85] |
| ZA01 (17), ZA02 (18) | antibacterial antifungal | Nocardiopsis SA 103 | non-rhizospheric soil samples, Saharan regions | [86] |
| Angucyclinone R2 (19) | antibacterial, antifungal, antitumor, antiviral, enzyme inhibitor, platelet aggregation inhibitor | Streptosporangium sp. Sg3 | Saharan soil, Adrar region | [87,88,89] |
| D(-)-threo chloramphenicol (20) | antibacterial | Saccharothrix sp. PAL54 | Saharan soil of Ghardaı¨a | [91] |
| 2-Hydroxy-5-((6-hydroxy-4-oxo-4H-pyran-2-yl)methyl)-2-propylchroman-4-one (21), Phaeochromycins B (22), C (23), E(24) | antibacterial, anti-inflammatory | Streptomyces sundarbansensis WR1L1S8 | Endophitic strain, inner tissue of marine algeae Fucus sp. | [53] |
| Compound A4 (25), Compound A5 (26) | anti-Gram-positive and -negative bacteria, anti-phytopathogenic and toxinogenic fungi | Saccharothrix SA198 | Saharan soil, Tamanrasset (Southern Algeria) | [93] |
| Saquayamycin A (27), Saquayamycin C (28) | antifungal and antibacterial | Streptomyces spp. PAL114 | Saharan soil, Béni-isguen-Ghardaïa (South of Algeria). | [94] |
| Vineomycin A1 (29), chaetoglobosin A (30) | antibacterial and antifungal | Streptomyces sp. PAL114. | Saharan soil | [95] |
| Novel hydroxamic acid (31) | antibacterial | Streptomyces WAB9 | Saharan soil, Bechar | [96] |
| Di-(2-ethylhexyl) phthalate (32) | antibacterial | Streptomyces sp. G60 | Saharan soil, Ghardaïa | [97] |
| 2,4-Di-tert-butylphenol (33) | against Candida albicans and other pathogenic fungi | Streptomyces mutabilis G61 | Soil sample Metlili, Ghardaïa | [99] |
| AT37-1 (34) | against multidrug-resistant S. aureus | Streptomyces sp. AT37 | Saharan soil sample (Adrar) | [100] |
| (−)-7-Deoxy-8-O-methyltetrangomycin (35), (−)-8-Methyltetrangomycin (36) | anti-Gram-positive bacteria, antifungal | Nocardiopsis sp. HR-4 | Salt-lake soil, Sebkha of Ain Salah (Saharan desert) | [103] |
| Streptazolin (37) | antimicrobial adjuvant antibiotic | Streptomyces thermoviolaceus SRC3 | Fresh water river sediments | [43] |
| Oligomycin A (NK1) (38), Oligomycin E (NK2) (39) | anti-Gram-positive bacteria, antifungal | Streptomyces sp. HG29 | Saharan soil sample (Hoggar, Tamanrasset) | [104] |
| Nigericin (40), Epinigericin (41), Abierixin (42), Grisorixin methyl ester (43) | glioblastoma stem-cell inhibitor | Streptomyces youssoufiensis SF10 | soil derived | [105] |
| Cyanogriside I (44), Cyanogriside J (45), Caerulomycin A (46), Caerulomycin F (47), Caerulomycinonitrile (48) | anti-Gram-positive bacteria, antifungal | Saccharothrix xinjiangensis ABH26 | Saharan soil (Adrar) | [107] |
| Actinomycin D (49) | antimicrobial, antitumor | Streptomyces sp. IA1. Streptomyces sp. GSBNT10 | Saharan soil (Ain amenas) Saharan soil (Beni Abbes-Bechar) | [110,111] |
| Indole-3-acetic acid (50) | plant-growth-promoting activity | Streptomyces sp. PT2 | Spontaneous herbaceous plants (Cleome arabica, Solanum nigrum, Astragallus armatus, Aristida pungens, and Panicum turgidum) (Sahara, Hassi R’mel region) | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djinni, I.; Defant, A.; Kecha, M.; Mancini, I. Actinobacteria Derived from Algerian Ecosystems as a Prominent Source of Antimicrobial Molecules. Antibiotics 2019, 8, 172. https://doi.org/10.3390/antibiotics8040172
Djinni I, Defant A, Kecha M, Mancini I. Actinobacteria Derived from Algerian Ecosystems as a Prominent Source of Antimicrobial Molecules. Antibiotics. 2019; 8(4):172. https://doi.org/10.3390/antibiotics8040172
Chicago/Turabian StyleDjinni, Ibtissem, Andrea Defant, Mouloud Kecha, and Ines Mancini. 2019. "Actinobacteria Derived from Algerian Ecosystems as a Prominent Source of Antimicrobial Molecules" Antibiotics 8, no. 4: 172. https://doi.org/10.3390/antibiotics8040172
APA StyleDjinni, I., Defant, A., Kecha, M., & Mancini, I. (2019). Actinobacteria Derived from Algerian Ecosystems as a Prominent Source of Antimicrobial Molecules. Antibiotics, 8(4), 172. https://doi.org/10.3390/antibiotics8040172