Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy
Abstract
:1. Introduction
1.1. Antimicrobial Treatment in Patients with CRS
- (1)
- Antibiotics are expected to alleviate the baseline symptoms of CRS, because they decrease the load of bacteria that may supposedly play a role in perpetuating the inflammation [6];
- (2)
- (3)
- Antibiotics eliminate bacteria that cause acute exacerbations of CRS (AECRS) i.e., acute infections that temporarily worsen the chronic symptoms.
1.2. Bacteriophage Therapy Versus Antibiotic Therapy for AECRS
- Increasing prevalence of antibiotic-resistant bacteria is observed in sinonasal infections worldwide;
- Biofilms that constitute a bacterial reservoir for recurrent exacerbations prove virtually impossible to eradicate with antibiotics;
- Non-selective elimination of both pathogenic and potentially beneficial bacteria caused by antibiotics results in uncontrolled repopulation of the empty niches. This process cannot be controlled and may not lead to restoration of an ‘optimal’ microbial community. The role of potential probiotics is still too poorly understood to prevent reintroduction of pathogenic species;
- Repeated courses of antibiotic therapy may contribute to increasing antibiotic resistance of the patient’s microbiota;
- In some patients, antibiotics cause serious adverse effects or allergic reactions.
- The mechanisms of antibiotic and phage resistance are entirely different. Therefore, bacterial strains that acquired antibiotic resistance frequently remain sensitive to phages;
- Some phages are able to penetrate and disrupt bacterial biofilms;
- The phages are highly selective. They eliminate only selected bacterial strains and leave the rest of the microbial community intact;
- Introduction of phage therapy instead of repeated antibiotic courses may prevent further selection of antibiotic-resistant strains;
- Phage preparations were shown to be generally safe and well-tolerated.
1.3. Aims of the Study
2. Results
3. Discussion
3.1. Microbiology and Antibiotic Resistance in AECRS
3.2. Phage Therapy for AECRS
3.3. Limitations of the Study
- Culture provides limited information compared to molecular methods that would allow for more profound analysis of the microbiota in AECRS [13]. Culture-dependent techniques were utilized in this study because they were required for phage typing, which was the essential part of the project.
- The current study did not include identification of anaerobes; further research is required to address this problem.
- Phage susceptibility testing was limited to the contents of the collection available for our study. However, the collection is still being developed and there is a possibility of phage isolation on demand.
- Further research is required to test the phage sensitivity of bacteria in biofilms and in polymicrobial communities.
4. Materials and Methods
4.1. Patient Recruitment
4.2. Specimen Collection
4.3. Bacterial Culture and Identification
4.4. Determination of Antibiotic Resistance
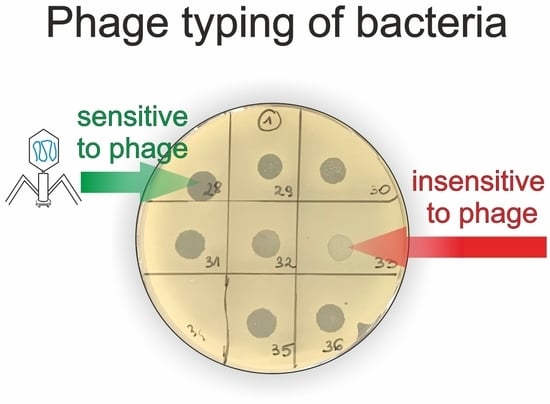
4.5. Phage Typing—Spot Test
- n—no clearing—no bacterial lysis in the spot;
- p—a few single plaques in the spot;
- o3—turbid spot—very weak bacterial lysis in the spot;
- o2—medium turbid spot—weak bacterial lysis in the spot;
- o1—almost clear spot—very weak bacterial growth in the spot;
- c—completely clear spot—complete bacterial lysis in the spot.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hastan, D.; Fokkens, W.J.; Bachert, C.; Newson, R.B.; Bislimovska, J.; Bockelbrink, A.; Bousquet, P.J.; Brozek, G.; Bruno, A.; Dahlen, S.E.; et al. Chronic rhinosinusitis in Europe—An underestimated disease. A GA(2)LEN study. Allergy 2011, 66, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, D.L.; Lucas, J.W.; Clarke, T.C. Summary health statistics for U.S. adults: National health interview survey, 2012. Vital Health Stat. 10 2014, 260, 1–161. [Google Scholar]
- Gliklich, R.E.; Metson, R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol. Head Neck Surg. 1995, 113, 104–109. [Google Scholar] [CrossRef]
- Bhattacharyya, N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. Ann. Otol. Rhinol. Laryngol. 2011, 120, 423–427. [Google Scholar] [CrossRef] [PubMed]
- DeConde, A.S.; Soler, Z.M. Chronic rhinosinusitis: Epidemiology and burden of disease. Am. J. Rhinol. Allergy 2016, 30, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.L.; Borish, L. Chronic rhinosinusitis and antibiotics: The good, the bad, and the ugly. Am. J. Rhinol. Allergy 2013, 27, 467–472. [Google Scholar] [CrossRef]
- Smith, S.S.; Evans, C.T.; Tan, B.K.; Chandra, R.K.; Smith, S.B.; Kern, R.C. National burden of antibiotic use for adult rhinosinusitis. J. Allergy Clin. Immunol. 2013, 132, 1230–1232. [Google Scholar] [CrossRef] [Green Version]
- Foreman, A.; Jervis-Bardy, J.; Wormald, P.J. Do biofilms contribute to the initiation and recalcitrance of chronic rhinosinusitis? Laryngoscope 2011, 121, 1085–1091. [Google Scholar] [CrossRef]
- Singhal, D.; Psaltis, A.J.; Foreman, A.; Wormald, P.J. The impact of biofilms on outcomes after endoscopic sinus surgery. Am. J. Rhinol. Allergy 2010, 24, 169–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Linkin, D.R.; Finkelman, B.S.; O’Malley, B.W.; Thaler, E.R.; Doghramji, L.; Kennedy, D.W.; Cohen, N.A.; Palmer, J.N. Asthma and biofilm-forming bacteria are independently associated with revision sinus surgeries for chronic rhinosinusitis. J. Allergy Clin. Immunol. 2011, 128, 221–223. [Google Scholar] [CrossRef]
- Hoggard, M.; Wagner Mackenzie, B.; Jain, R.; Taylor, M.W.; Biswas, K.; Douglas, R.G. Chronic rhinosinusitis and the evolving understanding of microbial ecology in chronic inflammatory mucosal disease. Clin. Microbiol. Rev. 2017, 30, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.R.; Hauser, L.J.; Frank, D.N. The sinonasal bacterial microbiome in health and disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boase, S.; Foreman, A.; Cleland, E.; Tan, L.; Melton-Kreft, R.; Pant, H.; Hu, F.Z.; Ehrlich, G.D.; Wormald, P.J. The microbiome of chronic rhinosinusitis: Culture, molecular diagnostics and biofilm detection. BMC Infect. Dis. 2013, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Barshak, M.B.; Durand, M.L. The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig. Otolaryngol. 2017, 2, 36–42. [Google Scholar] [CrossRef]
- Head, K.; Chong, L.Y.; Piromchai, P.; Hopkins, C.; Philpott, C.; Schilder, A.G.; Burton, M.J. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst. Rev. 2016, 4, CD011994. [Google Scholar] [CrossRef]
- Jain, R.; Douglas, R. When and how should we treat biofilms in chronic sinusitis? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 16–21. [Google Scholar] [CrossRef]
- Orlandi, R.R.; Kingdom, T.T.; Hwang, P.H.; Smith, T.L.; Alt, J.A.; Baroody, F.M.; Batra, P.S.; Bernal-Sprekelsen, M.; Bhattacharyya, N.; Chandra, R.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6 (Suppl. 1), S22–S209. [Google Scholar] [CrossRef]
- Brook, I. Bacteriology of chronic sinusitis and acute exacerbation of chronic sinusitis. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1099–1101. [Google Scholar] [CrossRef]
- Benninger, M.; Ferguson, B.; Hadley, J.; Hamilos, D.; Jacobs, M.; Kennedy, D.; Lanza, D.; Marple, B.; Osguthorpe, J.; Stankiewicz, J. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol. Head Neck Surg. 2003, 129, S1–S32. [Google Scholar] [CrossRef]
- Walgama, E.; Thanasumpun, T.; Gander, R.; Batra, P.S. Comparison of endoscopically-guided swab vs aspirate culture techniques in post-endoscopic sinus surgery patients: Blinded, prospective analysis. Int. Forum Allergy Rhinol. 2013, 3, 726–730. [Google Scholar] [CrossRef]
- Solares, C.A.; Batra, P.S.; Hall, G.S.; Citardi, M.J. Treatment of chronic rhinosinusitis exacerbations due to methicillin-resistant Staphylococcus aureus with mupirocin irrigations. Am. J. Otolaryngol. 2006, 27, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012, 50, 3, preceding table of contents, 1–298. [Google Scholar]
- Phillips, K.M.; Hoehle, L.P.; Bergmark, R.W.; Caradonna, D.S.; Gray, S.T.; Sedaghat, A.R. Acute exacerbations mediate quality of life impairment in chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2017, 5, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Hamilos, D.L. Are antibiotics useful for chronic rhinosinusitis? J. Allergy Clin. Immunol. Pract. 2016, 4, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S.T. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Miedzybrodzki, R.; Borysowski, J.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawelczyk, Z.; Rogoz, P.; Klak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83 Pt B, 73–121. [Google Scholar]
- Azeredo, J.; Sutherland, I.W. The use of phages for the removal of infectious biofilms. Curr. Pharm. Biotechnol. 2008, 9, 261–266. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar]
- Psaltis, A.J.; Li, G.; Vaezeafshar, R.; Cho, K.S.; Hwang, P.H. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope 2014, 124, 2216–2223. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Piccirillo, J.F.; Chandrasekhar, S.S.; Brook, I.; Ashok Kumar, K.; Kramper, M.; Orlandi, R.R.; Palmer, J.N.; Patel, Z.M.; Peters, A.; et al. Clinical practice guideline (update): Adult sinusitis. Otolaryngol. Head Neck Surg. 2015, 152, S1–S39. [Google Scholar] [CrossRef] [PubMed]
- Brook, I.; Foote, P.A.; Frazier, E.H. Microbiology of acute exacerbation of chronic sinusitis. Ann. Otol. Rhinol. Laryngol. 2005, 114, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.D.; Kennedy, D.W. Treatment options for chronic rhinosinusitis. Proc. Am. Thorac. Soc. 2011, 8, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.; Kepnes, L.J. The microbiology of recurrent rhinosinusitis after endoscopic sinus surgery. Arch. Otolaryngol. Head Neck Surg. 1999, 125, 1117–1120. [Google Scholar] [CrossRef]
- Yan, C.H.; Tangbumrungtham, N.; Maul, X.A.; Ma, Y.; Nayak, J.V.; Hwang, P.H.; Patel, Z.M. Comparison of outcomes following culture-directed vs. non-culture-directed antibiotics in treatment of acute exacerbations of chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2018, 8, 1028–1033. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Kou, Y.F.; Batra, P.S. Endoscopic culture-directed antibiotic therapy: Impact on patient symptoms in chronic rhinosinusitis. Am. J. Otolaryngol. 2015, 36, 642–646. [Google Scholar] [CrossRef]
- Cincik, H.; Ferguson, B.J. The impact of endoscopic cultures on care in rhinosinusitis. Laryngoscope 2006, 116, 1562–1568. [Google Scholar] [CrossRef]
- Kingdom, T.T.; Swain, R.E., Jr. The microbiology and antimicrobial resistance patterns in chronic rhinosinusitis. Am. J. Otolaryngol. 2004, 25, 323–328. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Kepnes, L.J. Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2008, 117, 448–452. [Google Scholar] [CrossRef]
- Szaleniec, J.; Gorski, A.; Szaleniec, M.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Strek, P.; Skladzien, J. Can phage therapy solve the problem of recalcitrant chronic rhinosinusitis? Future Microbiol. 2017, 12, 1427–1442. [Google Scholar] [CrossRef]
- Drilling, A.; Morales, S.; Jardeleza, C.; Vreugde, S.; Speck, P.; Wormald, P.J. Bacteriophage reduces biofilm of Staphylococcus aureus ex vivo isolates from chronic rhinosinusitis patients. Am. J. Rhinol. Allergy 2014, 28, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.A.; Drilling, A.; Morales, S.; Cornet, M.E.; Woodworth, B.A.; Fokkens, W.J.; Psaltis, A.J.; Vreugde, S.; Wormald, P.J. Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front. Cell. Infect. Microbiol. 2017, 7, 418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, Y.; Paramasivan, S.; Richter, K.; Morales, S.; Wormald, P.J.; Vreugde, S. Bacteriophage effectively kills multidrug resistant Staphylococcus aureus clinical isolates from chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2018, 8, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Drilling, A.; Morales, S.; Boase, S.; Jervis-Bardy, J.; James, C.; Jardeleza, C.; Tan, N.C.W.; Cleland, E.; Speck, P.; Vreugde, S.; et al. Safety and efficacy of topical bacteriophage and ethylenediaminetetraacetic acid treatment of Staphylococcus aureus infection in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2014, 4, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Drilling, A.J.; Ooi, M.L.; Miljkovic, D.; James, C.; Speck, P.; Vreugde, S.; Clark, J.; Wormald, P.J. Long-term safety of topical bacteriophage application to the frontal sinus region. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Fenton, M.; Casey, P.G.; Hill, C.; Gahan, C.G.M.; Ross, R.P.; McAuliffe, O.; O’Mahony, J.; Maher, F.; Coffey, A. The truncated phage lysin CHAP(k) eliminates Staphylococcus aureus in the nares of mice. Bioeng. Bugs 2010, 1, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Gupta, P.; Kaur, S. Bacteriophage as effective decolonising agent for elimination of MRSA from anterior nares of BALB/c mice. BMC Microbiol. 2014, 14. [Google Scholar] [CrossRef]
- Fong, S.A.; Drilling, A.J.; Ooi, M.L.; Paramasivan, S.; Finnie, J.W.; Morales, S.; Psaltis, A.J.; Vreugde, S.; Wormald, P.J. Safety and efficacy of a bacteriophage cocktail in an in vivo model of Pseudomonas aeruginosa sinusitis. Transl. Res. 2019, 206, 41–56. [Google Scholar] [CrossRef]
- Weber-Dabrowska, B.; Koźmińska, J.; Mulczyk, M.; Kaczkowski, H. The application of bacteriophages in the treatment of chronic purulent maxillary sinusitis. Post. Med. Klin. Dośw. 1996, 5, 291–293. [Google Scholar]
- Weber-Dabrowska, B.; Mulczyk, M.; Górski, A. Bacteriophage therapy of bacterial infections: An update on our institute’s experience. Arch. Immunol. Ther. Exp. (Warsz.) 2000, 48, 547–551. [Google Scholar]
- Speck, P.G.; Wormald, P.J. Is phage therapy suitable for treating chronic sinusitis Staphylococcus aureus infection? Future Microbiol. 2018, 13, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.L.; Drilling, A.J.; Morales, S.; Fong, S.; Moraitis, S.; Macias-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Nadel, D.M.; Lanza, D.C.; Kennedy, D.W. Endoscopically guided cultures in chronic sinusitis. Am. J. Rhinol. 1998, 12, 233–241. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing v 6.0.; EUCAST: Basel, Switzerland, 2017; Available online: http://www.eucast.org (accessed on 1 April 2018).
- Matuschek, E.; Brown, D.F.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical Breakpoints—Bacteria (v 8.0). Available online: www.eucast.org (accessed on 1 April 2018).
- Rak, G. New Strains of Bacteriophages, Specific to Bacteria Belonging to Pseudomonas genus and Their Application in Production of the Preparations Fighting Bacterial Infections. PL 228 848 B1, 24 June 2015. [Google Scholar]
- Rak, G. New Strains of Bacteriophages, Specific to Bacteria Belonging to Staphylococcus genus and Their Application in Production of the Preparations Fighting Bacterial Infections. PL 228 849 B1, 4 June 2015. [Google Scholar]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol. Biol. 2009, 501, 141–149. [Google Scholar] [PubMed]
- Dera-Tomaszewska, B.; Tokarska-Pietrzak, E. Phage types recognized within Salmonella enteritidis strains isolated in Poland in 1996–2007. Przegl. Epidemiol. 2012, 66, 611–616. [Google Scholar] [PubMed]
- Maszewska, A.; Wojcik, E.; Ciurzynska, A.; Wojtasik, A.; Piatkowska, I.; Dastych, J.; Rozalski, A. Differentiation of polyvalent bacteriophages specific to uropathogenic Proteus mirabilis strains based on the host range pattern and RFLP. Acta Biochim. Pol. 2016, 63, 303–310. [Google Scholar] [CrossRef]



| Gender | Male 23 (46%) Female 27 (54%) |
|---|---|
| Age | 25–80 (mean 51) |
| Nasal polyps | 45 (90%) |
| Comorbidities | |
| Asthma | 27 (54%) |
| Allergy (to pollen, dust mites, etc.) | 19 (38%) |
| Aspirin-exacerbated respiratory disease | 10 (20%) |
| Gastroesophageal reflux | 8 (16%) |
| History of CRS (years) | 1.5–45 (median 10) |
| History of recurrent exacerbations and repeated antibiotic treatment | 31 (62%) |
| Number of prior ESS procedures | 1–5 (median 1.5) |
| Time since the last ESS (months) | 1–96 (median 11) |
| Lund–Mackay computed tomography staging score [29] prior to surgery (total 0–24) | 6–24 (median 15) |
| Modified Lund–Kennedy endoscopic score [30] on enrollment (0–2 for polyps, edema, discharge on each side, total 0–12) | 2–12 (mean 6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaleniec, J.; Gibała, A.; Pobiega, M.; Parasion, S.; Składzień, J.; Stręk, P.; Gosiewski, T.; Szaleniec, M. Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy. Antibiotics 2019, 8, 175. https://doi.org/10.3390/antibiotics8040175
Szaleniec J, Gibała A, Pobiega M, Parasion S, Składzień J, Stręk P, Gosiewski T, Szaleniec M. Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy. Antibiotics. 2019; 8(4):175. https://doi.org/10.3390/antibiotics8040175
Chicago/Turabian StyleSzaleniec, Joanna, Agnieszka Gibała, Monika Pobiega, Sylwia Parasion, Jacek Składzień, Paweł Stręk, Tomasz Gosiewski, and Maciej Szaleniec. 2019. "Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy" Antibiotics 8, no. 4: 175. https://doi.org/10.3390/antibiotics8040175
APA StyleSzaleniec, J., Gibała, A., Pobiega, M., Parasion, S., Składzień, J., Stręk, P., Gosiewski, T., & Szaleniec, M. (2019). Exacerbations of Chronic Rhinosinusitis—Microbiology and Perspectives of Phage Therapy. Antibiotics, 8(4), 175. https://doi.org/10.3390/antibiotics8040175