Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori
Abstract
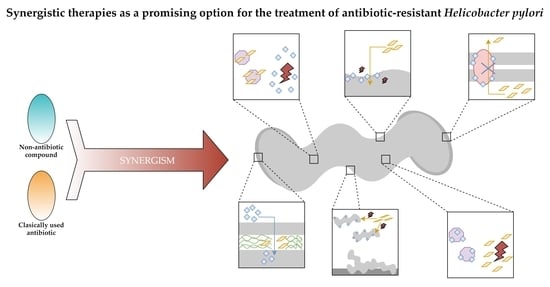
:1. Introduction
2. Review Strategy and Discussion
2.1. Chemically Synthesized Inorganic Compounds
Silver Ultra-Nanoclusters
2.2. Chemically Synthesized Organic Compounds
2.2.1. Auranofin
2.2.2. Sertraline
2.2.3. 3-Bromopyruvate
2.2.4. Niclosamide
2.2.5. Dihydropyridine Derivatives
2.3. Plant-Derived Organic Compounds and Plant Extracts
2.3.1. Flavonoids: Chrysin and Hesperetin
2.3.2. Artemisone
2.3.3. Pistacia vera Oleoresins
2.3.4. Citrus bergamia Juice
2.4. Mammal-Derived Organic Compounds
2.4.1. Lithocholic Acid
2.4.2. Bovine Lactoferrin
2.5. Microbes-Derived Organic Compounds
2.5.1. Pyrones and Their Derivatives
2.5.2. Rhamnolipids
3. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Pohl, D.; Keller, P.M.; Bordier, V.; Wagner, K. Review of Current Diagnostic Methods and Advances in Helicobacter pylori Diagnostics in the Era of Next Generation Sequencing. World J. Gastroenterol. 2019, 25, 4629–4660. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors that Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [Green Version]
- Domșa, A.-M.T.; Lupușoru, R.; Gheban, D.; Șerban, R.; Borzan, C.M. Helicobacter pylori Gastritis in Children—The Link between Endoscopy and Histology. J. Clin. Med. 2020, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- Soluri, M.F.; Puccio, S.; Caredda, G.; Edomi, P.; D’Elios, M.M.; Cianchi, F.; Troilo, A.; Santoro, C.; Sblattero, D.; Peano, C. Defining the Helicobacter pylori Disease-Specific Antigenic Repertoire. Front. Microbiol. 2020, 11, 1551. [Google Scholar] [CrossRef]
- Kao, C.Y.; Sheu, B.S.; Wu, J.J. Helicobacter pylori Infection: An Overview of Bacterial Virulence Factors and Pathogenesis. Biomed. J. 2016, 39, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zhu, Y.; Lu, N.H.; Shi, Q. Recent Progress in Helicobacter pylori Treatment. Chin. Med. J. 2020, 133, 335–343. [Google Scholar] [CrossRef]
- Saleem, N.; Howden, C.W. Update on the Management of Helicobacter pylori Infection. Curr. Treat. Options Gastroenterol. 2020, 1–12. [Google Scholar] [CrossRef]
- Boyanova, L.; Hadzhiyski, P.; Kandilarov, N.; Markovska, R.; Mitov, I. Multidrug Resistance in Helicobacter pylori: Current State and Future Directions. Expert Rev. Clin. Pharmacol. 2019, 12, 909–915. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, C.; Chen, Z.; Xu, Z.; Li, H.; Li, W.; Sun, Y. Transporters HP0939, HP0497, and HP0471 Participate in Intrinsic Multidrug Resistance and Biofilm Formation in Helicobacter pylori by Enhancing Drug Efflux. Helicobacter 2020, 25, e12715. [Google Scholar] [CrossRef]
- Fauzia, K.A.; Miftahussurur, M.; Syam, A.F.; Waskito, L.A.; Doohan, D.; Rezkitha, Y.A.A.; Matsumoto, T.; Tuan, V.P.; Akada, J.; Yonezawa, H.; et al. Biofilm Formation and Antibiotic Resistance Phenotype of Helicobacter pylori Clinical Isolates. Toxins 2020, 12, 473. [Google Scholar] [CrossRef]
- Krzyżek, P.; Grande, R. Transformation of Helicobacter pylori into Coccoid Forms as a Challenge for Research Determining Activity of Antimicrobial Substances. Pathogens 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of Biofilm Formation by Quorum Quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Sutton, P.; Boag, J.M. Status of Vaccine Research and Development for Helicobacter pylori. Vaccine 2019, 37, 7295–7299. [Google Scholar] [CrossRef]
- Rizvanov, A.A.; Abadi, A.T.B. Helicobacter pylori Infection and Vaccination: Current Standoff. Bionanoscience 2019, 9, 928–929. [Google Scholar] [CrossRef]
- Losurdo, G.; Cubisino, R.; Barone, M.; Principi, M.; Leandro, G.; Ierardi, E.; Di Leo, A. Probiotic Monotherapy and Helicobacter pylori Eradication: A Systematic Review with Pooled-Data Analysis. World J. Gastroenterol. 2018, 24, 139–149. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, X.; Liu, X.; Song, Y.P.; Song, C.; Wu, S.; An, Y.; Yuan, R.; Wang, Y.; Xie, Y. The Effect of Antibiotic Resistance on Helicobacter pylori Eradication Efficacy: A Systematic Review and Meta-Analysis. Helicobacter 2020, 25, e12714. [Google Scholar] [CrossRef]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Combination Approaches to Combat Multidrug-Resistant Bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Tyers, M.; Wright, G.D. Drug Combinations: A Strategy to Extend the Life of Antibiotics in the 21st Century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug Evolutionary Strategies to Reverse Antibiotic Resistance. Science 2016, 351, aad3292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.; Azim, A.; Gurjar, M.; Baronia, A.K. Current Concepts in Combination Antibiotic Therapy for Critically Ill Patients. Indian J. Crit. Care Med. 2014, 18, 310–314. [Google Scholar] [PubMed] [Green Version]
- Pavicic, M.J.A.M.P.; Namavar, F.; Verboom, T.; Van Winkelhoff, A.J.; De Graaff, J. In Vitro Susceptibility of Helicobacter pylori to Several Antimicrobial Combinations. Antimicrob. Agents Chemother. 1993, 37, 1184–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cederbrant, G.; Kahlmeter, G.; Schatén, C.; Kamme, C. Additive Effect of Clarithromycin Combined with 14-hydroxy Clarithromycin, Erythromycin, Amoxycillin, Metronidazole or Omeprazole against Helicobacter pylori. J. Antimicrob. Chemother. 1994, 34, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M. Antibacterial Properties of Lansoprazole Alone and in Combination with Antimicrobial Agents against Helicobacter pylori. J. Clin. Gastroenterol. 1995, 20, S32–S37. [Google Scholar] [CrossRef]
- Alarcón, T.; Domingo, D.; Sánchez, I.; Rojas, F.D.; López-Brea, M. In Vitro Activity of Omeprazole in Combination with Several Antimicrobial Agents against Clinical Isolates of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 937–940. [Google Scholar] [CrossRef]
- Van Caekenberghe, D.L.; Breyssens, J. In Vitro Synergistic Activity between Bismuth Subcitrate and Various Antimicrobial Agents against Campylobacter pyloridis (C. pyloridis). Antimicrob. Agents Chemother. 1987, 31, 1429–1430. [Google Scholar] [CrossRef] [Green Version]
- Vogt, K.; Hahn, H. Synergism between Clindamycin and Colloidal Bismuth Sub citrate against Helicobacter (Campylobacter) pylori In Vitro. Zentralblatt fur Bakteriol. 1990, 274, 246–249. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lee, S.Y. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol. Clin. N. Am. 2015, 44, 537–563. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, R.; Sisto, F.; Puca, V.; Carradori, S.; Ronci, M.; Aceto, A.; Muraro, R.; Mincione, G.; Scotti, L. Antimicrobial and Antibiofilm Activities of New Synthesized Silver Ultra-NanoClusters (SUNCs) against Helicobacter pylori. Front. Microbiol. 2020, 11, 1705. [Google Scholar] [CrossRef] [PubMed]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial Activity and Mechanism of Action of Auranofin against Multi-Drug Resistant Bacterial Pathogens. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin Exerts Broad-Spectrum Bactericidal Activities by Targeting Thiol-Redox Homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs R D 2015, 15, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Wiederhold, N.P.; Patterson, T.F.; Srinivasan, A.; Chaturvedi, A.K.; Fothergill, A.W.; Wormley, F.L.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. Repurposing Auranofin as an Antifungal: In Vitro Activity against a Variety of Medically Important Fungi. Virulence 2017, 8, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Owings, J.; McNair, N.; Mui, Y.; Gustafsson, T.; Holmgren, A.; Contel, M.; Goldberg, J.; Mead, J. Auranofin and N-heterocyclic Carbene Gold-Analogs Are Potent Inhibitors of the Bacteria Helicobacter pylori. FEMS Microbiol. Lett. 2016, 363, fnw148. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.S.; Martinez-de-Oliveira, J.; Donders, G.G.G.; Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A. Anti-Candida Activity of Antidepressants Sertraline and Fluoxetine: Effect upon Pre-formed Biofilms. Med. Microbiol. Immunol. 2018, 207, 195–200. [Google Scholar] [CrossRef]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The Antidepressant Sertraline Provides a Promising Therapeutic Option for Neurotropic Cryptococcal Infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, M.L.; Abengózar, M.A.; Nácher-Vázquez, M.; Martínez-Alcázar, M.P.; Barbas, C.; Tempone, A.G.; López-Gonzálvez, Á.; Rivas, L. Molecular Basis of the Leishmanicidal Activity of the Antidepressant Sertraline as a Drug Repurposing Candidate. Antimicrob. Agents Chemother. 2018, 62, e01928-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weeks, J.C.; Roberts, W.M.; Leasure, C.; Suzuki, B.M.; Robinson, K.J.; Currey, H.; Wangchuk, P.; Eichenberger, R.M.; Saxton, A.D.; Bird, T.D.; et al. Sertraline, Paroxetine, and Chlorpromazine Are Rapidly Acting Anthelmintic Drugs Capable of Clinical Repurposing. Sci. Rep. 2018, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.-U.; Ullah, F.; Ullah, I.; Ali, G.; Syed, N.-I.-H.; Hussain, S. Sertraline Enhances the Activity of Antimicrobial Agents Against Pathogens of Clinical Relevance. J. Biol. Res. 2015, 22, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In Vitro Activity of Sertraline, an Antidepressant, Against Antibiotic-Susceptible and Antibiotic-Resistant Helicobacter pylori Strains. Pathogens 2019, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- de Lima, L.P.O.; Seabra, S.H.; Carneiro, H.; Barbosa, H.S. Effect of 3-Bromopyruvate and Atovaquone on Infection during In Vitro Interaction of Toxoplasma gondii and LLC-MK2 Cells. Antimicrob. Agents Chemother. 2015, 59, 5239–5249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Boradia, V.M.; Thakare, R.; Singh, A.K.; Gani, Z.; Das, S.; Patidar, A.; Dasgupta, A.; Chopra, S.; Raje, M.; et al. Repurposing Ethyl Bromopyruvate as a Broad-Spectrum Antibacterial. J. Antimicrob. Chemother. 2019, 74, 912–920. [Google Scholar] [CrossRef]
- Cal, M.; Matyjaszczyk, I.; Litwin, I.; Augustyniak, D.; Ogórek, R.; Ko, Y.; Ułaszewski, S. The Anticancer Drug 3-Bromopyruvate Induces DNA Damage Potentially Through Reactive Oxygen Species in Yeast and in Human Cancer Cells. Cells 2020, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In Vitro Activity of 3-Bromopyruvate, an Anti-Cancer Compound, Against Antibiotic-Susceptible and Antibiotic-Resistant Helicobacter pylori Strains. Cancers 2019, 11, 229. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Mook, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an Antihelminthic Drug. Cell. Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Xu, J.; Shi, P.Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Imperi, F.; Massai, F.; Pillai, C.R.; Longo, F.; Zennaro, E.; Rampioni, G.; Visc, P.; Leoni, L. New Life for an Old Drug: The Anthelmintic Drug Niclosamide Inhibits Pseudomonas aeruginosa Quorum Sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, C.; Burgain, A.; Chaillot, J.; Pic, É.; Khemiri, I.; Sellam, A. A Phenotypic Small-Molecule Screen Identifies Halogenated Salicylanilides as Inhibitors of Fungal Morphogenesis, Biofilm Formation and Host Cell Invasion. Sci. Rep. 2018, 8, 11559. [Google Scholar] [CrossRef] [Green Version]
- Tharmalingam, N.; Port, J.; Castillo, D.; Mylonakis, E. Repurposing the Anthelmintic Drug Niclosamide to Combat Helicobacter pylori. Sci. Rep. 2018, 8, 3701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, T.S.; El-Sayed, H.A.; Khayat, M.T.; AL-Mahmoudy, A.M.M.; Moustafa, A.H.; El-Deen, A.K.S.; Rostom, S.A.F.; Panda, S.S. Synthesis of Nucleosides and Non-nucleosides Based 4,6-disubstituted-2- oxo-dihydropyridine-3-carbonitriles as Antiviral Agents. Med. Chem. (Los. Angeles) 2018, 14, 791–808. [Google Scholar] [CrossRef]
- Olejníková, P.; Švorc, L.; Olšovská, D.; Panáková, A.; Vihonská, Z.; Kovaryová, K.; Marchalín, Š. Antimicrobial Activity of Novel C2-Substituted 1,4-Dihydropyridine Analogues. Sci. Pharm. 2014, 82, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhillar, A.K.; Arya, P.; Mukherjee, C.; Kumar, P.; Yadav, Y.; Sharma, A.K.; Yadav, V.; Gupta, J.; Dabur, R.; Jha, H.N.; et al. Microwave-Assisted Synthesis of Antimicrobial Dihydropyridines and Tetrahydropyrimidin-2-ones: Novel Compounds against Aspergillosis. Bioorg. Med. Chem. 2006, 14, 973–981. [Google Scholar] [CrossRef]
- Maya, J.D.; Morello, A.; Repetto, Y.; Tellez, R.; Rodriguez, A.; Zelada, U.; Puebla, P.; Caballero, E.; Medarde, M.; Núñez-Vergara, L.J.; et al. Effects of 3-Chloro-phenyl-1,4-dihydropyridine Derivatives on Trypanosome cruzi Epimastigotes. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2000, 125, 103–109. [Google Scholar] [CrossRef]
- Velena, A.; Zarkovic, N.; Gall Troselj, K.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1,4-Dihydropyridine Derivatives: Dihydronicotinamide Analogues-Model Compounds Targeting Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Casado, J.; Chueca, E.; Salillas, S.; Velázquez-Campoy, A.; Angarica, V.E.; Bénejat, L.; Guignard, J.; Giese, A.; Sancho, J.; et al. Repurposing Dihydropyridines for Treatment of Helicobacter pylori Infection. Pharmaceutics 2019, 11, 681. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef] [Green Version]
- Aboody, M.S.A.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising Natural Compounds against Viral Infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Lehane, A.M.; Saliba, K.J. Common Dietary Flavonoids Inhibit the Growth of the Intraerythrocytic Malaria Parasite. BMC Res. Notes 2008, 1, 26. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Salillas, S.; Velázquez-Campoy, A.; Espinosa Angarica, V.; Fillat, M.F.; Sancho, J.; Lanas, Á. Identifying Potential Novel Drugs against Helicobacter pylori by Targeting the Essential Response Regulator HsrA. Sci. Rep. 2019, 9, 11294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coertzen, D.; Reader, J.; Van Der Watt, M.; Nondaba, S.H.; Gibhard, L.; Wiesner, L.; Smith, P.; D’Alessandro, S.; Taramelli, D.; Wong, H.N.; et al. Artemisone and Artemiside are Potent Panreactive Antimalarial Agents that Also Synergize Redox Imbalance in Plasmodium Falciparum Transmissible Gametocyte Stages. Antimicrob. Agents Chemother. 2018, 62, e02214-17. [Google Scholar] [CrossRef] [Green Version]
- Oiknine-Djian, E.; Weisblum, Y.; Panet, A.; Wong, H.N.; Haynes, R.K.; Wolfa, D.G. The Artemisinin Derivative Artemisone is a Potent Inhibitor of Human Cytomegalovirus Replication. Antimicrob. Agents Chemother. 2018, 62, e00288-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, P.; Upadhyay, S.K.; Hassan, W.; Madan, T.; Sirdeshmukh, R.; Sundaram, C.S.; Gade, W.N.; Basir, S.F.; Singh, Y.; Sarma, P.U. Transcriptomic and Proteomic Profile of Aspergillus fumigatus on Exposure to Artemisinin. Mycopathologia 2011, 172, 331–346. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Cui, H. Antibacterial Mechanism of Artemisinin / Beta-Cyclodextrins against Methicillin-Resistant Staphylococcus aureus (MRSA). Microb. Pathog. 2018, 118, 66–73. [Google Scholar] [CrossRef]
- Sisto, F.; Scaltrito, M.M.; Masia, C.; Bonomi, A.; Coccè, V.; Marano, G.; Haynes, R.K.; Miani, A.; Farronato, G.; Taramelli, D. In Vitro Activity of Artemisone and Artemisinin Derivatives against Extracellular and Intracellular Helicobacter pylori. Int. J. Antimicrob. Agents 2016, 48, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Filocamo, A.; Faulks, R.M.; Mandalari, G. In Vitro Antimicrobial Activity of Pistachio (Pistacia vera L.) Polyphenols. FEMS Microbiol. Lett. 2013, 341, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napoli, E.; Gentile, D.; Ruberto, G. GC-MS Analysis of Terpenes from Sicilian Pistacia vera L. Oleoresin. A Source of Biologically Active Compounds. Biomed. Chromatogr. 2019, 33, e4381. [Google Scholar] [CrossRef]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia Species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. Sci. World J. 2013, 2013, 219815. [Google Scholar] [CrossRef] [Green Version]
- Di Lodovico, S.; Napoli, E.; Di Campli, E.; Di Fermo, P.; Gentile, D.; Ruberto, G.; Nostro, A.; Marini, E.; Cellini, L.; Di Giulio, M. Pistacia vera L. Oleoresin and Levofloxacin is a Synergistic Combination against Resistant Helicobacter pylori Strains. Sci. Rep. 2019, 9, 4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauman, M.C.; Johnson, J.J. Clinical Application of Bergamot (Citrus bergamia) for Reducing High Cholesterol and Cardiovascular Disease Markers. Integr. Food, Nutr. Metab. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [Green Version]
- Fratianni, F.; Cozzolino, A.; de Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò Cultivated in Southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial Activity of Flavonoids Extracted from Bergamot (Citrus bergamia Risso) Peel, a Byproduct of the Essential Oil Industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Pizzimenti, F.; Ferlazzo, A.; Giofr, S.V.; Iannazzo, D.; Piperno, A.; Romeo, R.; Chiacchio, M.A.; Mastino, A.; MacChi, B. Antiviral Activity of Seed Extract from Citrus bergamia Towards Human Retroviruses. Bioorganic Med. Chem. 2011, 19, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In Vitro Effect of Bergamot (Citrus bergamia) Juice against CagA-positive and -negative Clinical Isolates of Helicobacter pylori. BMC Complement. Altern. Med. 2015, 15, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pols, T.W.H.; Puchner, T.; Korkmaz, H.I.; Vos, M.; Soeters, M.R.; de Vries, C.J.M. Lithocholic Acid Controls Adaptive Immune Responses by Inhibition of Th1 Activation through the Vitamin D Receptor. PLoS ONE 2017, 12, e0176715. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Rahman, M.A.; Mercado, L.; Shokfai, T.; Decho, A.W.; Reineke, T.M.; Tang, C. Facially Amphiphilic Polyionene Biocidal Polymers Derived from Lithocholic Acid. Bioact. Mater. 2018, 3, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Sannasiddappa, T.H.; Lund, P.A.; Clarke, S.R. In Vitro Antibacterial Activity of Unconjugated and Conjugated Bile Salts on Staphylococcus aureus. Front. Microbiol. 2017, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.E.; Donaldson, J.R. Effect of Bile Salts on the DNA and Membrane Integrity of Enteric Bacteria. J. Med. Microbiol. 2009, 58, 1533–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinan, J.; Villa, P.; Thangamani, S. Secondary Bile Acids Inhibit Candida albicans Growth and Morphogenesis. Pathog. Dis. 2018, 76, 38. [Google Scholar] [CrossRef] [Green Version]
- Schupp, A.-K.; Trilling, M.; Rattay, S.; Le-Trilling, V.T.K.; Haselow, K.; Stindt, J.; Zimmermann, A.; Häussinger, D.; Hengel, H.; Graf, D. Bile Acids Act as Soluble Host Restriction Factors Limiting Cytomegalovirus Replication in Hepatocytes. J. Virol. 2016, 90, 6686–6698. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Casado, J.; Chueca, E.; Salillas, S.; Velázquez-Campoy, A.; Sancho, J.; Lanas, Á. Small Molecule Inhibitors of the Response Regulator ArsR Exhibit Bactericidal Activity against Helicobacter pylori. Microorganisms 2020, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A Natural Antimicrobial Protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef] [Green Version]
- Kutila, T.; Pyörälä, S.; Saloniemi, H.; Kaartinen, L. Antibacterial Effect of Bovine Lactoferrin against Udder Pathogens. Acta Vet. Scand. 2003, 44, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e02284-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccaglione, A.F.; Di Giulio, M.; Di Lodovico, S.; Di Campli, E.; Cellini, L.; Marzio, L. Bovine Lactoferrin Enhances the Efficacy of Levofloxacin-Based Triple Therapy as First-Line Treatment of Helicobacter pylori Infection: An in Vitro and in Vivo Study. J. Antimicrob. Chemother. 2019, 74, 1069–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choque, E.; El Rayess, Y.; Raynal, J.; Mathieu, F. Fungal Naphtho-γ-Pyrones—Secondary Metabolites of Industrial Interest. Appl. Microbiol. Biotechnol. 2015, 99, 1081–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-Naphtho-γ-Pyrones from Fungi and Their Bioactivities. Molecules 2014, 19, 7169–7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, X.; Jia, J.; Xue, Y.; Ding, W.; Dong, Z.; Tian, D.; Chen, M.; Bi, H.; Hong, K.; Tang, J. New Pyrones and their Analogs from the Marine Mangrove-Derived Aspergillus sp. DM94 with Antibacterial Activity against Helicobacter pylori. Appl. Microbiol. Biotechnol. 2020, 104, 7971–7978. [Google Scholar] [CrossRef]
- He, Y.; Tian, J.; Chen, X.; Sun, W.; Zhu, H.; Li, Q.; Lei, L.; Yao, G.; Xue, Y.; Wang, J.; et al. Fungal Naphtho-γ-Pyrones: Potent Antibiotics for Drug-Resistant Microbial Pathogens. Sci. Rep. 2016, 6, 24291. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Yang, X.; Li, Z.; Fang, Z.; et al. Effects of Rhamnolipids on Microorganism Characteristics and Applications in Composting: A Review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef]
- Ndlovu, T.; Rautenbach, M.; Vosloo, J.A.; Khan, S.; Khan, W. Characterisation and Antimicrobial Activity of Biosurfactant Extracts Produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa Isolated from a Wastewater Treatment Plant. AMB Express 2017, 7, 108. [Google Scholar] [CrossRef] [Green Version]
- Gaur, V.K.; Tripathi, V.; Gupta, P.; Dhiman, N.; Regar, R.K.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Patel, D.K.; Manickam, N. Rhamnolipids from Planococcus spp. and Their Mechanism of Action against Pathogenic Bacteria. Bioresour. Technol. 2020, 307, 123206. [Google Scholar] [CrossRef]
- Remichkova, M.; Galabova, D.; Roeva, I.; Karpenko, E.; Shulga, A.; Galabov, A.S. Anti-Herpesvirus Activities of Pseudomonas sp. S-17 Rhamnolipid and Its Complex with Alginate. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2008, 63, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, P.; Shen, Y.; Zou, Y.; Yuan, G.; Hu, H. Rhamnolipid-involved Antibiotics Combinations Improve the Eradication of Helicobacter pylori Biofilm In Vitro: A Comparison with Conventional Triple Therapy. Microb. Pathog. 2019, 131, 112–119. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Structure | Chemical Characteristic | Application | Antimicrobial Spectrum | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|
| Silver ultra-nanoclusters |  | Chemically synthesized inorganic compounds | Components of bionanomaterials, antimicrobial preparations, dressings, and cover surfaces | Bacteria, fungi, parasites, viruses |
| [30,31,32,33,34] |
| Auranofin |  | Chemically synthesized organogold compounds | Rheumatoid arthritis drug | Bacteria, fungi, parasites, viruses |
| [35,36,37,38,39] |
| Sertraline |  | Chemically synthesized organic compounds | Antidepressant drug | Bacteria, fungi, parasites, viruses |
| [40,41,42,43,44,45] |
| 3-bromopyruvate |  | Chemically synthesized organic compounds | Anticancer agent | Bacteria, fungi, parasites |
| [46,47,48,49] |
| Niclosamide |  | Chemically synthesized organic compounds | Antiparasitic drug | Bacteria, fungi, parasites, viruses |
| [50,51,52,53,54] |
| Dihydropyridine derivatives |  Nisoldipine | Chemically synthesized organic compounds | Hypertension drugs | Bacteria, fungi, parasites, viruses |
| [55,56,57,58,59,60] |
| Flavonoids: chrysin and hesperetin |  Chrysin  Hesperetin | Plant-derived organic compounds | Antioxidant and anti-inflammatory compounds | Bacteria, fungi, parasites, viruses |
| [61,62,63,64,65,66] |
| Artemisone |  | Plant-derived organic compounds | Antiparasitic drug (anti-Plasmodium agent) | Bacteria, fungi, parasites, viruses |
| [67,68,69,70,71] |
| Pistacia vera oleoresins |
Main components: Dipterocarpol
Dipterocarpol Masticadienonic acid | Plant-derived organic compounds | Used in ethnomedicine for gastrointestinal complaints | Bacteria, fungi, parasites, viruses |
| [72,73,74,75] |
| Citrus bergamia juice |
Main components: Neoeriocitrin
Neoeriocitrin Neohesperidin | Plant extract | Used in ethnomedicine for diabetes and cardiovascular disorders | Bacteria, fungi, viruses |
| [76,77,78,79,80,81] |
| Lithocholic acid (3α-hydroxy-5β-cholan-24-oic acid) |  | Mammal-derived organic compounds | Natural components of biliary system with digestive, antimicrobial, and immunomodulatory properties | Bacteria, fungi, viruses |
| [82,83,84,85,86,87,88] |
| Bovine lactoferrin |  | Mammal-derived organic compounds | Natural components of immune system with antimicrobial and immunomodulatory properties | Bacteria, fungi, parasites, viruses |
| [89,90,91,92,93] |
| Pyrones and their derivatives |  Aurasperone A | Microbe-derived organic compounds | Modulators of hormones | Bacteria, fungi, viruses |
| [94,95,96,97] |
| Rhamnolipids |  | Microbe-derived organic compounds | Antiadhesive and antibiofilm agents | Bacteria, fungi, viruses |
| [98,99,100,101,102] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżek, P.; Paluch, E.; Gościniak, G. Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori. Antibiotics 2020, 9, 658. https://doi.org/10.3390/antibiotics9100658
Krzyżek P, Paluch E, Gościniak G. Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori. Antibiotics. 2020; 9(10):658. https://doi.org/10.3390/antibiotics9100658
Chicago/Turabian StyleKrzyżek, Paweł, Emil Paluch, and Grażyna Gościniak. 2020. "Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori" Antibiotics 9, no. 10: 658. https://doi.org/10.3390/antibiotics9100658
APA StyleKrzyżek, P., Paluch, E., & Gościniak, G. (2020). Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori. Antibiotics, 9(10), 658. https://doi.org/10.3390/antibiotics9100658