MeJA Elicitation of Chicory Hairy Roots Promotes Efficient Increase of 3,5-diCQA Accumulation, a Potent Antioxidant and Antibacterial Molecule
Abstract
:1. Introduction
2. Results
2.1. Establishment of the HRCs and Growth Parameters in Liquid Medium
2.2. Improvement of Specialized Metabolite Production
2.3. Tri-Caffeoylquinic Acid Production in Chicory Hairy Roots
2.4. Purification and Structural Identification of the Major Isomers of di-CQA and tri-CQA
2.5. Antimicrobial Activity and CQAs Quantification
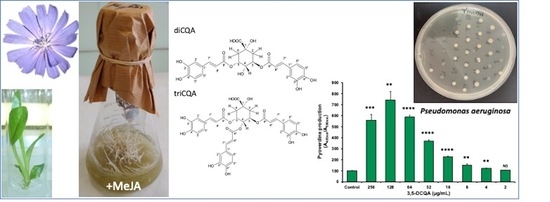
2.6. Effect of 3,5-Dicaffeoylquinic Acid on Pseudomonas aeruginosa Virulence Factors Production and Biofilm Formation
2.7. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Rhizobium Strain
4.2. Establishment of HRC and Molecular Confirmation of Their Phenotype
4.3. Measurement of HRC Growth
4.4. Elicitation and Scale Up
4.5. Extraction and Analysis of Polyphenols
4.6. Extraction and Purification of 3,5-Dicaffeoylquinic Acid (di-CQA) and 3,4,5-Tricaffeoylquinic Acid (tri-CQA)
4.7. UHPLC-UV-MS Analyzes and Structural Identification by NMR
4.8. Extraction for Bioassays and Quantification of CQAs
4.9. Antibacterial Screening of Extracts, Sub-Extracts and Pure Compounds Using Agar Dilution Method
4.10. Pyocyanin and Pyoverdine Quantification Assays
4.11. Quantitative Biofilm Assay
4.12. Statistical Analyzes
4.13. Antioxidant Activity Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Legrand, G.; Delporte, M.; Khelifi, C.; Hardant, A.; Vuylsteker, C.; Mörchen, M.; Hance, P.; Hilbert, J.-L.; Gagneul, D. Identification and characterization of five BAHD acyltransferases involved in hydroxycinnamoyl ester metabolism in chicory. Front. Plant. Sci. 2016, 7, 741. [Google Scholar] [CrossRef]
- Cadalen, T.; Mörchen, M.; Blassiau, C.; Clabaut, A.; Scheer, I.; Hilbert, J.-L.; Hendriks, T.; Quillet, M.C. Development of SSR markers and construction of a consensus genetic map for chicory (Cichorium intybus L.). Mol. Breed. 2010, 25, 699–722. [Google Scholar] [CrossRef]
- Nandagopal, S.; Ranjhita Kumari, B.D. Phytochemical and antibacterial studies of chicory (Cichorium intybus L.)–A multipurpose medicinal plant. Adv. Biol. Res. 2007, 1, 17–21. [Google Scholar]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Vasudeva, N.; Sharma, S. Cichorium intybus: A concise report on its ethnomedicinal, botanical, and phytopharmacological aspects. Drug Dev. Ther. 2016, 7, 1–12. [Google Scholar]
- Bahri, M.; Hance, P.; Grec, S.; Quillet, M.-C.; Trotin, F.; Hilbert, J.-L.; Hendriks, T. A “novel” protocol for the analysis of hydroxycinnamic acids in leaf tissue of chicory (Cichorium intybus L., Asteraceae). Sci. World J. 2012, 2012, 142983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willeman, H.; Hance, P.; Fertin, A.; Voedts, N.; Duhal, N.; Goossens, J.-F.; Hilbert, J.-L. A method for simultaneous determination of chlorogenic acid and sesquiterpene lactone content in industrial chicory root foodstuffs. Sci. World J. 2014, 2014, 583180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Besseau, S.; Geoffroy, P.; Ritzenthaler, C.; Meyer, D.; Lapierre, C.; Pollet, B.; Legrand, M. Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant. Cell 2004, 16, 1446–1465. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Tan, F.; Yang, J.; Yang, Y.; Gou, Y.; Li, S.; Zhao, X. Antioxidant effects of Apocynum venetum tea extracts on d-galactose-induced aging model in mice. Antioxidants 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheleva-Dimitrova, D.; Gevrenova, R.; Zaharieva, M.M.; Najdenski, H.; Ruseva, S.; Lozanov, V.; Balabanova, V.; Yagi, S.; Momekov, G.; Mitev, V. HPLC-UV and LC–MS Analyses of Acylquinic Acids in Geigeria alata (DC) Oliv. & Hiern. and their Contribution to Antioxidant and Antimicrobial Capacity. Phytochem. Anal. 2017, 28, 176–184. [Google Scholar]
- Wan, P.; Xie, M.; Chen, G.; Dai, Z.; Hu, B.; Zeng, X.; Sun, Y. Anti-inflammatory effects of dicaffoylquinic acids from Ilex kudingcha on lipopolysaccharide-treated RAW264.7 macrophages and potential mechanisms. Food Chem. Toxicol. 2019, 126, 332–342. [Google Scholar] [CrossRef]
- Zhou, M.L.; Zhu, X.-M.; Shao, J.-R.; Tang, Y.-X.; Wu, Y.-M. Production and metabolic engineering of bioactive substances in plant hairy root culture. Appl. Microbial. Biotechnol. 2011, 90, 1229–1239. [Google Scholar] [CrossRef]
- Ono, N.N.; Tian, L. The multiplicity of hairy root cultures: Prolific possibilities. Plant. Sci. 2011, 180, 439–446. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, R. Engineering secondary metabolite production in hairy roots. Phytochem. Rev. 2011, 10, 371–395. [Google Scholar] [CrossRef]
- Tsygankova, V.A.; Yemets, A.I.; Ponomarenko, S.P.; Matvieieva, N.A.; Chapkevich, S.E.; Kuchuk, N.V. Increase in the synthesis of polyfructan in the cultures of chicory “hairy roots” with plant natural growth regulators. Int. J. Biomed. 2013, 3, 139–144. [Google Scholar]
- Fathi, R.; Mohebodini, M.; Chamani, E. High-efficiency Agrobacterium rhizogenes-mediated genetic transformation in Cichorium intybus L. via removing macronutrients. Ind. Crop. Prod. 2019, 128, 572–580. [Google Scholar] [CrossRef]
- Bais, H.P.; Sudha, G.; Ravishankar, G.A. Putrescine influences growth and production of coumarins in hairy root cultures of witloof chicory (Cichorium intybus L. cv. Lucknow local). J. Plant. Growth Regul. 1999, 18, 159–165. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Long-term cultures hairy roots of chicory—A rich source of hydroxycinnamates and 8-deoxylactucin glucoside. Appl. Biochem. Biotechnol. 2013, 171, 1589–1601. [Google Scholar] [CrossRef] [Green Version]
- Bogdanovic, M.D.; Todorovic, S.I.; Banjanac, T.; Drajicevic, M.B.; Verstappen, F.W.A.; Bouwmeester, H.J.; Simonovic, A.D. Production of guaianolides in Agrobacterium rhizogenes- transformed chicory regenerants flowering in vitro. Ind. Crop. Prod. 2014, 6052–6059. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Sesquiterpene lactones in a hairy root culture of Cichorium intybus. Z. Nat. C 2002, 57, 994–997. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Li, S.; Liu, L.; Chen, C.; Fan, S. Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L. Plants 2017, 6, 10. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Yu, M.; Zhang, Q.I.; Wu, G.; Li, S.; Yu, J.; Hu, Y. Application of accelerated solvent extraction coupled with high-performance counter-current chromatography to extraction and online isolation of chemical constituents from Hypericum perforatum L. J. Chromatogr. A 2011, 1218, 2827–2834. [Google Scholar] [CrossRef]
- Kono, Y.; Kashine, S.; Yonekama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Lima, J.P. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Z.; Abbasi, B.H.; Gao, M.; Murch, S.J.; Saxena, P.K. Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J. Agric. Food Chem. 2006, 54, 8456–8460. [Google Scholar] [CrossRef] [PubMed]
- Stojakowska, A.; Malarz, J.; Szewczyk, A.; Kisiel, W. Caffeic acid derivatives from a hairy root culture of Lactuca virosa. Acta Physiol. Plant. 2012, 34, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Skala, E.; Kicel, A.; Olszewska, M.A.; Kiss, A.K.; Wysokinska, H. Establishment of hairy root cultures of Rhaponticum carthamoides (Willd.) Iljin for the production of biomass and caffeic acid derivatives. BioMed. Res. Int. 2015, 2015, 181098. [Google Scholar] [CrossRef] [Green Version]
- Kundu, S.; Salma, U.; Ali, M.N.; Hazra, A.K.; Mandal, N. Development of transgenic hairy roots and augmentation of secondary metabolites by precursor feeding in Sphagneticola calendulacea (L.) Pruski. Ind. Crops Prod. 2018, 121, 206–215. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Thiruvengadam, M.; Chung, I.-M. Identification of elicitors enhances the polyphenolic compounds and pharmacological potential in hairy root cultures of Aster scaber. S. Afr. J. Bot. 2019, 125, 92–101. [Google Scholar] [CrossRef]
- Azam Ansari, M.; Chung, I.-M.; Rajakumar, G.; Alzohairy, M.A.; Almatroudi, A.; Khanna, V.G.; Thiruvengadam, M. Evaluation of polyphenolic compounds and pharmacological activities in hairy root cultures of Ligularia fischeri Turca. F. spiciformis (Nakai). Molecules 2019, 24, 1586. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, V.R.; Krekling, T.; Christiansen, E. Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am. J. Bot. 2002, 89, 578–586. [Google Scholar] [CrossRef]
- Gundlach, H.; Müller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 1992, 89, 2389–2393. [Google Scholar] [CrossRef] [Green Version]
- Amani, S.; Mohebodini, M.; Khademvatan, S.; Jafari, M. Agrobacterium rhizogenes mediated transformation of Ficus carica L. for the efficient production of secondary metabolites. J. Sci. Food Agric. 2020, 100, 2185–2197. [Google Scholar] [CrossRef]
- Malarz, J.; Stojakowska, A.; Kisiel, W. Effect of methyl jasmonate and salicylic acid on sesquiterpene lactone accumulation in hairy roots of Cichorium intybus. Acta Physiol. Plant. 2007, 29, 127–132. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase a inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, K.; Pan, T.; Liu, J.; Li, B.; Li, C.; Wang, S.; Diao, Y.; Liu, X. Lonicerin, an anti-algE flavonoid against Pseudomonas aeruginosa virulence screened from Shuanghuanglian formula by molecule docking based strategy. J. Ethnopharmacol. 2019, 239, 111909. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Ye, C.; Gaeta, B.; Tao, H.; Wang, M.; Qiu, Z. Chlorogenic acid attenuates virulence factors and pathogenicity of Pseudomonas aeruginosa by regulating quorum sensing. Appl. Microbiol. Biotechnol. 2019, 103, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Sconamiglio, M.; Buommino, E.; Coretti, L.; Graziani, V.; Russo, R.; Caputo, P.; Donnarumma, G.; D’Abrosca, B.; Fiorentino, A. Phytochemical investigation and antimicrobial assessment of Bellis sylvestris leaves. Phytochem. Lett. 2016, 17, 6–13. [Google Scholar] [CrossRef]
- Cerulli, A.; Lauro, G.; Masullo, M.; Cantone, V.; Olas, B.; Kontek, B.; Nazzaro, F.; Bifulco, G.; Piacente, S. Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities. J. Nat. Prod. 2017, 80, 1703–1713. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Muscolo, C.; Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Vitali, L.A.; Vittori, S.; Maggi, F. Bioactive Secondary Metabolites from Schizogyne sericea (Asteraceae) Endemic to Canary Islands. Chem. Biodivers. 2016, 13, 826–836. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Hubert, J.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Renault, J.H.; Nuzillard, J.M.; Morjani, H.; Abedini, A.; Gangloff, S.C.; et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018, 125, 41–48. [Google Scholar] [CrossRef]
- Crone, S.; Vives-Flórez, M.; Kvich, L.; Saunders, A.M.; Malone, M.; Nicolaisen, M.H.; Martínez-García, E.; Rojas-Acosta, C.; Gomez-Puerto, M.C.; Calum, H.; et al. The environmental occurrence of Pseudomonas aeruginosa. Apmis 2020, 128, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Sommer, L.M.; Johansen, H.K.; Molin, S. Antibiotic resistance in Pseudomonas aeruginosa and adaptation to complex dynamic environments. Microb Genom. 2020, 6. [Google Scholar] [CrossRef]
- Tahrioui, A.; Duchesne, R.; Bouffartigues, E.; Rodrigues, S.; Maillot, O.; Tortuel, D.; Hardouin, J.; Taupin, L.; Groleau, M.C.; Dufour, A.; et al. Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. NPJ Biofilms Microbiomes 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahrioui, A.; Ortiz, S.; Azuama, O.C.; Bouffartigues, E.; Benalia, N.; Tortuel, D.; Maillot, O.; Chemat, S.; Kritsanida, M.; Feuilloley, M.; et al. Membrane-interactive compounds from Pistachia lentiscus L. thwart Pseudomonas aeruginosa virulence. Front. Microbiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Heller, R. Recherches sur la nutrition minérale des tissus végétaux cultivés in vitro. Ann. Sci. Nat. Bot. Biol. Vég. 1953, 14, 1–223. [Google Scholar]
- Vervliet, G.; Holsters, M.; Teuchy, H.; Van Montagu, M.; Schell, J. Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. J. Gen. Virol. 1975, 26, 33–48. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Haas, J.H.; Moore, L.W.; Ream, W.; Manulis, S. Universal PCR primers for detection of phytopathogenic Agrobacterium strains. Appl. Environ. Microbiol. 1995, 6, 2879–2884. [Google Scholar] [CrossRef] [Green Version]
- Serino, G.; Clerot, D.; Brevet, J.; Costantino, P.; Cardarelli, M. Rol genes of Agrobacterium rhizogenes cucumopine strain: Sequence, effects and pattern expression. Plant. Mol. Biol. 1994, 26, 415–422. [Google Scholar] [CrossRef]
- Delporte, M.; Bernard, G.; Legrand, G.; Hielscher, B.; Lanoue, A.; Molinié, R.; Rambaud, C.; Mathiron, D.; Besseau, S.; Linka, N.; et al. A BAHD neofunctionalization promotes tetrahydoxycinnamoyl spermine accumulation in pollen coat of the Asteraceae family. J. Exp. Bot. 2018, 69, 5355–5371. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic compounds from Humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant Staphylococcus aureus, Leishmania mexicalna and Trypanosoma brucei strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Taofic, O.; Gonzales-Paramas, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R. Hydroxycinnamic Acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J.-W. Antioxidant and anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated from Ligularia fischeri leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]








| Hairy Root Crude Extracts and Sub-Extracts | Quantification of CQAs (mg.g−1) | |||
|---|---|---|---|---|
| CQA | di-CQA | tri-CQA | ||
| HR1 | Crude methanolic | 10.01 | 45.01 | 0.3 |
| H2O | 8.70 | 3.43 | 0 | |
| EtOAc | 21.80 | 452.15 | 1.58 | |
| HR2 | Crude methanolic | 10.05 | 69.70 | 1.45 |
| H2O | 10.65 | 6.91 | 0 | |
| EtOAc | 18.07 | 508.5 | 8.68 | |
| Bacterial and Fungal Pathogen Strains | MIC (mg mL−1) | MIC (µg mL−1) | |||||
|---|---|---|---|---|---|---|---|
| EtOAc HR1 | EtOAc HR2 | CQA | di-CQA | GEN | VAN | AMX | |
| Gram positive | |||||||
| Corynebacterium striatum T40A3 | 0.313 | 0.313 | 0.156 | 0.156 | 0.03 | 1 | 0.25 |
| Enterococcus faecalis C159-6 | NA | 1.25 | 0.625 | 0.625 | 4 | 0.5 | 64 |
| Enterococcus sp. 8153 | NA | NA | NA | NA | 2 | 4 | 2 |
| Staphylococcus aureus 8146 | 0.625 | 0.625 | 0.625 | 0.313 | 0.5 | 2 | 4 |
| Staphylococcus aureus 8241 | 0.625 | 0.313 | 0.313 | 0.313 | 0.5 | 2 | 16 |
| Staphylococcus aureus ATCC 6538 | 0.625 | 0.625 | 0.313 | 0.313 | 0.25 | 2 | 0.125 |
| Staphylococcus aureus T28-1 | 0.625 | 0.625 | 0.313 | 0.156 | 0.5 | 2 | 16 |
| Staphylococcus aureus T17-4 | 0.625 | 0.313 | 0.313 | 0.313 | 0.5 | 2 | 1 |
| Staphylococcus warneri T12A12 | 0.313 | 0.313 | 0.156 | 0.156 | 0.06 | 2 | 1 |
| Staphylococcus warneri T26A1 | 0.313 | 0.313 | 0.156 | 0.156 | 0.06 | 2 | 0.25 |
| Staphylococcus epidermidis T46A1 | 0.313 | 0.156 | 0.156 | 0.156 | 0.06 | 2 | 0.5 |
| Staphylococcus epidermidis T19A1 | 0.313 | 0.156 | 0.156 | 0.156 | 32 | 2 | 8 |
| Staphylococcus epidermidis T21A5 | 0.156 | 0.156 | 0.156 | 0.156 | 0.06 | 2 | 16 |
| Staphylococcus pettenkoferi T47A6 | 0.156 | 0.156 | 0.156 | 0.156 | 0.06 | 2 | 0.25 |
| Streptococcus agalactiae T53C9 | 0.625 | 0.313 | 0.313 | 0.156 | 1 | 0.5 | 0.03 |
| Streptococcus pyogenes 16138 | 0.313 | 0.156 | 0.313 | 0.313 | 0.125 | 0.25 | 0.03 |
| Gram negative | |||||||
| Citrobacter freundii 11041 | NA | NA | NA | 1.25 | 0.25 | >64 | 2 |
| Enterobacter aerogenes 9004 | NA | NA | NA | NA | 0.5 | >64 | >64 |
| Escherichia coli T20A1 | NA | NA | NA | NA | 0.25 | >64 | >64 |
| Escherichia coli 8138 | NA | NA | NA | NA | 0.5 | >64 | >64 |
| Escherichia coli 8157 | NA | NA | NA | NA | 0.5 | >64 | >64 |
| Escherichia coli ATCC 25922 | NA | NA | NA | NA | 0.5 | >64 | 16 |
| Klebsiella pneumoniae 11016 | NA | NA | NA | NA | 0.25 | >64 | >64 |
| Klebsiella pneumoniae 10270 | NA | NA | NA | NA | 8 | >64 | >64 |
| Proteus mirabilis 11060 | 1.25 | 0.625 | 0.625 | 0.625 | 0.5 | >64 | 2 |
| Proteus mirabilis T28-3 | 0.625 | 0.625 | 1.25 | 0.313 | 0.5 | >64 | 1 |
| Pseudomonas aeruginosa 8131 | 0.313 | 0.625 | 0.625 | 0.625 | 1 | >64 | >64 |
| Pseudomonas aeruginosa ATCC 27583 | 0.156 | 0.313 | 0.313 | 0.156 | 2 | >64 | >64 |
| Pseudomonas aeruginosa 8129 | 0.313 | 0.313 | 0.313 | 0.313 | 0.03 | >64 | >64 |
| Salmonella sp. 11033 | NA | NA | NA | NA | 0.25 | >64 | 2 |
| Fungi | AMB | FLC | SER | ||||
| Candida albicans 10286 | NA | 0.625 | NA | 1.25 | 4 | 32 | >64 |
| Candida albicans ATCC 10231 | 0.156 | 0.156 | 0.078 | 0.078 | 0.5 | 8 | 64 |
| Samples | IC50 (µM) | 50 (µM/µmol DPPH) | Antiradical Power (nM/µmol DPPH) | IC50 (µg/mL) | EC50 (µg/mL/µg DPPH) | Antiradical Power (µg/mL/µg DPPH) |
|---|---|---|---|---|---|---|
| crude methanolic extract HR1 | - | - | - | 89.96 | 1.17 | 0.86 |
| ethyl acetate sub-extract HR1 | - | - | - | 24.06 | 0.31 | 3.20 |
| aqueous sub-extract HR1 | - | - | - | 345.32 | 4.49 | 0.22 |
| crude methanolic extract HR2 | - | - | - | 52.76 | 0.69 | 1.46 |
| ethyl acetate sub-extract HR2 | - | - | - | 18.40 | 0.24 | 4.18 |
| aqueous sub-extract HR2 | - | - | - | 217.90 | 2.83 | 0.35 |
| 3-caffeoylquinic acid (CQA) | 37.70 | 193.32 | 5.17 | 13.35 | 0.17 | 5.76 |
| 3,5-dicaffeoylquinic acid (di-CQA) | 16.03 | 82.20 | 12.17 | 8.28 | 0.11 | 9.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, G.; Alves Dos Santos, H.; Etienne, A.; Samaillie, J.; Neut, C.; Sahpaz, S.; Hilbert, J.-L.; Gagneul, D.; Jullian, N.; Tahrioui, A.; et al. MeJA Elicitation of Chicory Hairy Roots Promotes Efficient Increase of 3,5-diCQA Accumulation, a Potent Antioxidant and Antibacterial Molecule. Antibiotics 2020, 9, 659. https://doi.org/10.3390/antibiotics9100659
Bernard G, Alves Dos Santos H, Etienne A, Samaillie J, Neut C, Sahpaz S, Hilbert J-L, Gagneul D, Jullian N, Tahrioui A, et al. MeJA Elicitation of Chicory Hairy Roots Promotes Efficient Increase of 3,5-diCQA Accumulation, a Potent Antioxidant and Antibacterial Molecule. Antibiotics. 2020; 9(10):659. https://doi.org/10.3390/antibiotics9100659
Chicago/Turabian StyleBernard, Guillaume, Harmony Alves Dos Santos, Audrey Etienne, Jennifer Samaillie, Christel Neut, Sevser Sahpaz, Jean-Louis Hilbert, David Gagneul, Nathalie Jullian, Ali Tahrioui, and et al. 2020. "MeJA Elicitation of Chicory Hairy Roots Promotes Efficient Increase of 3,5-diCQA Accumulation, a Potent Antioxidant and Antibacterial Molecule" Antibiotics 9, no. 10: 659. https://doi.org/10.3390/antibiotics9100659
APA StyleBernard, G., Alves Dos Santos, H., Etienne, A., Samaillie, J., Neut, C., Sahpaz, S., Hilbert, J. -L., Gagneul, D., Jullian, N., Tahrioui, A., Chevalier, S., Rivière, C., & Rambaud, C. (2020). MeJA Elicitation of Chicory Hairy Roots Promotes Efficient Increase of 3,5-diCQA Accumulation, a Potent Antioxidant and Antibacterial Molecule. Antibiotics, 9(10), 659. https://doi.org/10.3390/antibiotics9100659