Chemical Profiling and Discrimination of Essential Oils from Six Ferula Species Using GC Analyses Coupled with Chemometrics and Evaluation of Their Antioxidant and Enzyme Inhibitory Potential
Abstract
:1. Introduction
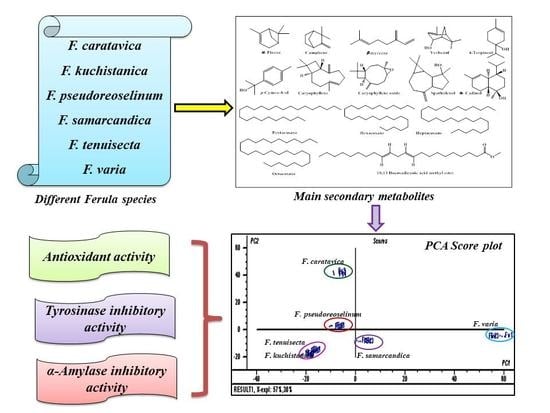
2. Results and Discussion
2.1. Qualitative and Semi-quantitative Determinations by GC-MS and GC-FID
2.2. Chemometric Analysis
2.3. Biological Evaluation
2.3.1. Antioxidant Potential of Different Ferula Species
2.3.2. Tyrosinase and α-Amylase Inhibitory Potential
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Essential Oil Samples
3.3. GC-FID and GC-MS Analyses
3.4. Chemometric and ANOVA Analysis
3.5. Biological Evaluation
3.5.1. Determination of the Antioxidant Potential
3.5.2. Determination of Enzyme Inhibitory Effects
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Biological activities of essential oils from the genus Ferula (Apiaceae). Asian Biomed. Res. Rev. News. 2010, 4, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.d.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Lee, S.E.; Hwang, H.J.; Ha, J.-S.; Jeong, H.-S.; Kim, J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef]
- Moniruzzaman; Shahinuzzaman; Haque, A.; Khatun, R.; Yaakob, Z. Gas chromatography mass spectrometry analysis and in vitro antibacterial activity of essential oil from Trigonella foenum-graecum. Asian Pac. J. Trop. Biomed. 2015, 5, 1033–1036. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flav. Frag. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Wang, P.; Li, L.; Qu, W.; Liang, J. Antimicrobial, antioxidant and cytotoxic properties of essential oil from Dictamnus angustifolius. J. Ethnopharmacol. 2015, 159, 296–300. [Google Scholar] [CrossRef]
- Martins, C.D.M.; Nascimento, E.A.D.; de Morais, S.A.; de Oliveira, A.; Chang, R.; Cunha, L.; Martins, M.M.; Martins, C.H.G.; Moraes, T.D.S.; Rodrigues, P.V. Chemical constituents and evaluation of antimicrobial and cytotoxic activities of Kielmeyera coriacea Mart. & Zucc. essential oils. Evid.-Based Complement. Altern. Med. 2015, 2015, 842047. [Google Scholar]
- Safari, O.; Sarkheil, M.; Paolucci, M. Dietary administration of Ferula (Ferula asafoetida) powder as a feed additive in diet of koi carp, Cyprinus carpio koi: Effects on hemato-immunological parameters, mucosal antibacterial activity, digestive enzymes, and growth performance. Fish. Physiol. Biochem. 2019, 45, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Sarker, S.D.; Nahar, L.; Akbarzadeh, A. The genus Ferula: Ethnobotany, phytochemistry and bioactivities—A review. Ind. Crops Prod. 2019, 129, 350–394. [Google Scholar] [CrossRef]
- Iranshahy, M.; Iranshahi, M. Traditional uses, phytochemistry and pharmacology of Asafoetida (Ferula assafoetida oleo-gum-resin)—A review. J. Ethnopharmacol. 2011, 134, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Him, A.; Ozbek, H.; Turel, I.; Oner, A.C. Antinociceptive activity of alpha-pinene and fenchone. Pharmacologyonline 2008, 3, 363–369. [Google Scholar]
- Amiri, H. Chemical composition and antioxidant activity of essential oil and methanolic extracts of Ferula microcolea (Boiss.) Boiss (Apiaceae). Int. J. Food Prop. 2014, 17, 722–730. [Google Scholar] [CrossRef]
- Nguir, A.; Mabrouk, H.; Douki, W.; Ismail, M.B.; Jannet, H.B.; Flamini, G. Chemical composition and bioactivities of the essential oil from different organs of Ferula communis L. growing in Tunisia. Med. Chem. Res. 2016, 25, 515–525. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-J.; Pan, Z.-Z.; Chen, C.-Q.; Hu, Y.-H.; Liu, F.-J.; Shi, Y.; Yan, J.-H.; Chen, Q.-X. Inhibitory effects of fatty acids on the activity of mushroom tyrosinase. Appl. Biochem Biotechnol. 2010, 162, 1564–1573. [Google Scholar] [CrossRef] [Green Version]
- Taherkhani, M. Chemical constituents, total phenolic content, antimicrobial, antioxidant and radical scavenging properties, chelating ability, tyrosinase inhibition and in vitro cytotoxic effects of Artemisia aucheri herbs. Pharm. Chem. J. 2017, 50, 736–745. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Ebada, S.S.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Antihyperglycaemic activity of the methanol extract from leaves of Eremophila maculata (Scrophulariaceae) in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2017, 69, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; El-Beshbishy, H.A.; Singab, A.N.B. Validation of the antihyperglycaemic and hepatoprotective activity of the flavonoid rich fraction of Brachychiton rupestris using in vivo experimental models and molecular modelling. Food Chem. Toxicol. 2018, 114, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Jelenkovic, L.; Jovanovic, V.S.; Palic, I.; Mitic, V.; Radulovic, M. In vitro screening of α-amylase inhibition by selected terpenes from essential oils. Trop. J. Pharm. Res. 2014, 13, 1421–1428. [Google Scholar] [CrossRef] [Green Version]
- Heydari-Majd, M.; Rezaeinia, H.; Shadan, M.R.; Ghorani, B.; Tucker, N. Enrichment of zein nanofibre assemblies for therapeutic delivery of Barije (Ferula gummosa Boiss) essential oil. J. Drug Deliv. Sci. Technol. 2019, 54, 101290. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Ashour, M.L.; Akramov, D.K.; Sasmakov, S.A.; Ramazonov, N.S.; Azimova, S.S. A comparative study on chemical composition and antimicrobial activity of essential oils from three Phlomis species from Uzbekistan. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Youssef, F.S.; Hamoud, R.; Ashour, M.L.; Singab, A.N.; Wink, M. Volatile oils from the aerial parts of Eremophila maculata and their antimicrobial activity. Chem. Biodivers. 2014, 11, 831–841. [Google Scholar] [CrossRef]
- Thabet, A.A.; Youssef, F.S.; El-Shazly, M.; Singab, A.N.B. GC-MS and GC-FID analyses of the volatile constituents of Brachychiton rupestris and Brachychiton discolor, their biological activities and their differentiation using multivariate data analysis. Nat. Prod. Res. 2020, 34, 590–594. [Google Scholar] [CrossRef]
- Labib, R.; Youssef, F.; Ashour, M.; Abdel-Daim, M.; Ross, S. Chemical composition of Pinus roxburghii bark volatile oil and validation of its anti-inflammatory activity using molecular modelling and bleomycin-induced inflammation in albino mice. Molecules 2017, 22, 1384. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Ashour, M.L.; Sasmakov, S.A.; Tiezzi, A.; Azimova, S.S. Chemical composition, antimicrobial and antioxidant activities of the essential oils of three Uzbek Lamiaceae species. Nat. Prod. Res. 2019, 33, 2394–2397. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Youssef, F.S.; El-Shazly, M.; Ashour, M.L.; Singab, A.N.B.; Wink, M. Volatile constituents of Dietes bicolor (Iridaceae) and their antimicrobial activity. Z. Naturforsch. C 2015, 70, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Sarker, S.D.; Nahar, L.; Al-Azizi, M.M.; Ashour, M.L. Authentication and discrimination of green tea samples using UV–vis, FTIR and HPLC techniques coupled with chemometrics analysis. J. Pharm. Biomed. Anal. 2019, 164, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.Z.; Böhmdorfer, S.; Zengin, G.; Bacher, M.; Potthast, A.; Akramov, D.K.; Janibekov, A.; Rosenau, T. Phytochemical and biological activities of Silene viridiflora extractives. Development and validation of a HPTLC method for quantification of 20-hydroxyecdysone. Ind. Crop. Prod. 2019, 129, 542–548. [Google Scholar] [CrossRef]





| Compound | RI | Content, (%) | Identification Methods | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cal. | Rep. | Fc | Fk | Fp | Fs | Ft | Fv | |||
| 1. | n-Nonane | 889 | 900 | 0.37 | - | 0.16 | - | - | - | MS, RI, |
| 2. | Tricyclene | 913 | 913 | - | - | - | - | 0.37 | - | MS, RI, |
| 3. | 3-Thujene | 919 | 919 | - | - | 0.50 | - | 0.36 | - | MS, RI, AU |
| 4. | α-Pinene | 925 | 925 | 21.17 | 36.79 | 10.99 | 1.5 | 42.0 | - | MS, RI, AU |
| 5. | Camphene | 941 | 941 | 2.91 | 0.47 | - | - | 8.34 | - | MS, RI, |
| 6. | Sabinene | 970 | 970 | - | - | 0.94 | - | 0.35 | - | MS, RI, |
| 7. | β-Pinene | 973 | 973 | 0.82 | 1.88 | 3.11 | - | 3.59 | - | MS, RI, AU |
| 8. | 6-Methyl-5-heptene-2-one | 986 | 986 | - | 0.35 | - | - | - | - | MS, RI, AU |
| 9. | β-Myrcene | 989 | 989 | - | 0.19 | 6.04 | 10.75 | 1.90 | - | MS, RI, |
| 10. | n-Decane | 998 | 1000 | 1.00 | 0.16 | 0.47 | 2.44 | 0.06 | 0.62 | MS, RI, |
| 11. | α-Phellandrene | 1003 | 1003 | - | 1.81 | tr | - | - | - | MS, RI, AU |
| 12. | (3E)-3-Hexenyl acetate | 1007 | 1006 | - | - | - | - | - | 1.40 | MS, RI, |
| 13. | 3-Carene | 1009 | 1009 | - | tr | 0.97 | - | 0.07 | - | MS, RI, |
| 14. | 2-Carene | 1016 | 1018 | - | - | tr | - | - | MS, RI, | |
| 15. | β-Cymene | 1024 | 1025 | - | tr | 2.59 | - | 0.39 | - | MS, RI, |
| 16. | Limonene | 1028 | 1028 | - | 1.77 | 0.83 | 1.15 | 4.80 | - | MS, RI, AU |
| 17. | τ-Terpinene | 1059 | 1059 | - | - | 0.30 | - | - | - | MS, RI, AU |
| 18. | Linalool oxide | 1074 | 1074 | - | 0.25 | - | - | - | - | MS, RI, |
| 19. | Terpinolene | 1089 | 1089 | - | - | - | - | tr | - | MS, RI, |
| 20. | Dehydro-p-cymene | 1090 | 1090 | - | - | - | - | 0.15 | - | MS, RI, |
| 21. | n-Undecane | 1098 | 1100 | tr | 1.60 | - | 1.56 | - | 0.43 | MS, RI, |
| 22. | β-Linalool | 1100 | 1100 | tr | tr | 2.03 | - | 0.54 | - | MS, RI, AU |
| 23. | cis-p-Menth-2,8-dienol | 1108 | 1104 | - | 0.80 | 1.78 | - | 0.40 | - | MS, RI, |
| 24. | Fenchol | 1116 | 1117 | - | - | - | - | 0.04 | - | MS, RI, |
| 25. | 6-Camphenol | 1128 | 1131 | - | 2.75 | - | 0.05 | - | MS, RI, | |
| 26. | Limonene oxide | 1135 | 1133 | - | - | - | - | tr | - | MS, RI, |
| 27. | 4-Isopropenyl-1-methyl-2-cyclohexen-1-ol | 1137 | 1142 | - | 0.32 | 0.49 | - | 0.12 | - | MS, RI, |
| 28. | L-pinocarveol | 1141 | 1141 | - | 3.90 | 0.74 | - | 0.33 | - | MS, RI, |
| 29. | Verbenol | 1148 | 1148 | - | 8.49 | - | - | 1.25 | - | MS, RI, |
| 30. | trans-2-Nonenal | 1160 | 1161 | - | - | - | - | 0.03 | 0.73 | MS, RI, |
| 31. | 3-Pinanone | 1163 | 1160 | - | tr | - | - | - | - | MS, RI, |
| 32. | Verbenone | 1165 | 1173 | - | 1.43 | - | - | - | - | MS, RI, |
| 33. | Borneol | 1169 | 1169 | - | - | - | - | 0.11 | - | MS, RI, |
| 34. | 4-Terpineol | 1179 | 1179 | - | 0.39 | 16.28 | - | 0.36 | - | MS, RI, |
| 35. | p-Cymen-8-ol | 1186 | 1186 | tr | 2.85 | 5.36 | - | 0.80 | 0.49 | MS, RI, |
| 36. | α-Terpineol | 1193 | 1193 | - | 0.52 | 5.00 | - | 0.41 | - | MS, RI, |
| 37. | Myrtenol | 1199 | 1199 | - | 2.30 | 0.65 | - | 0.21 | - | MS, RI, |
| 38. | cis-Geraniol | 1210 | 1210 | - | - | 0.35 | - | tr | - | MS, RI, |
| 39. | Verbenone | 1214 | 1214 | - | 3.95 | 1.11 | - | 0.18 | - | MS, RI, |
| 40. | Fenchyl acetate | 1222 | 1223 | - | 4.51 | - | - | - | - | MS, RI, |
| 41. | cis-Carveol | 1225 | 1220 | - | tr | - | - | 0.46 | - | MS, RI, |
| 42. | β-Citronellol | 1230 | 1230 | - | - | - | - | 0.05 | - | MS, RI, |
| 43. | trans-Carveol | 1234 | 1229 | - | - | - | - | 0.04 | - | MS, RI, |
| 44. | Thymol methyl ether | 1237 | 1237 | - | 0.19 | 0.23 | - | 0.03 | - | MS, RI, |
| 45. | D-Carvone | 1249 | 1249 | - | 1.68 | - | - | tr | - | MS, RI, |
| 46. | Nerol | 1252 | 1251 | - | tr | - | - | tr | - | MS, RI, |
| 47. | Bornyl acetate | 1290 | 1290 | - | 0.49 | 0.39 | - | 0.31 | - | MS, RI, |
| 48. | (-)-trans-Pinocarvyl acetate | 1305 | 297 | - | - | 1.19 | - | - | - | MS, RI, |
| 49. | Carvacrol | 1306 | 1306 | - | tr | - | - | - | - | MS, RI, |
| 50. | α-Cubebene | 1353 | 1353 | - | - | - | - | 0.16 | - | MS, RI, |
| 51. | D-longifolene | 1370 | 1370 | - | tr | - | - | - | - | MS, RI, |
| 52. | α-Copaene | 1380 | 1380 | - | 0.38 | 0.41 | - | 0.18 | 0.39 | MS, RI, |
| 53. | β-Gurjunene | 1386 | 1388 | - | 4.81 | - | - | - | - | MS, RI, |
| 54. | β-Bourbonene | 1390 | 1390 | - | - | - | - | - | tr | MS, RI, |
| 55. | β-Elemene | 1395 | 1395 | - | - | - | - | 0.24 | tr | MS, RI, |
| 56. | Jasmone | 1403 | 1399 | - | tr | - | - | - | - | MS, RI, |
| 57. | β-Caryophyllene | 1425 | 1425 | 10.88 | - | 0.91 | - | 0.08 | tr | MS, RI, AU |
| 58. | τ-Elemene | 1438 | 1438 | - | - | - | - | 0.12 | - | MS, RI, |
| 59. | Patchoulene | 1440 | 1440 | tr | - | 0.38 | - | - | MS, RI, | |
| 60. | Alloaromadendrene | 1447 | 1442 | - | - | - | - | - | 0.63 | MS, RI, |
| 61. | Geranyl acetone | 1456 | 1455 | - | 4.48 | 0.58 | - | - | - | MS, RI, |
| 62. | α-Humulene | 1461 | 1461 | 2.98 | tr | - | - | 0.04 | - | MS, RI, |
| 63. | τ-Muurolene | 1467 | 1467 | - | 0.27 | - | - | 0.21 | 0.87 | MS, RI, |
| 64. | α-Curcumene | 1487 | 1486 | - | tr | - | - | - | MS, RI, | |
| 65. | Germacrene D | 1489 | 1489 | - | - | - | - | 0.13 | - | MS, RI, |
| 66. | β-Eudesmene | 1495 | 1495 | - | - | - | - | 0.20 | - | MS, RI, |
| 67. | β-Guaiene | 1503 | 1500 | 0.65 | - | tr | - | 0.38 | tr | MS, RI, |
| 68. | α-Muurolene | 1508 | 1508 | - | - | 0.35 | - | 0.74 | - | MS, RI, |
| 69. | Cuparene | 1514 | 1513 | 3.09 | 0.23 | - | - | - | - | MS, RI, |
| 70. | α-Selinene | 1514 | 1517 | - | tr | - | - | 0.07 | 0.34 | MS, RI, |
| 71. | τ-Cadinene | 1523 | 1521 | - | - | - | - | 0.75 | 0.50 | MS, RI, |
| 72. | β-Cadinene | 1524 | 1529 | tr | - | - | - | - | - | MS, RI, |
| 73. | δ-Cadinene | 1531 | 1531 | 1.37 | - | - | tr | 3.07 | - | MS, RI, |
| 74. | Elemol | 1557 | 1577 | - | - | - | - | tr | - | MS, RI, |
| 75. | Nerolidol | 1566 | 1564 | 1.70 | - | - | - | 1.74 | - | MS, RI, |
| 76. | Germacrene B | 1572 | 1569 | - | - | - | - | - | 1.07 | MS, RI, |
| 77. | Germacrene D-4-ol | 1585 | 1583 | - | - | - | - | 0.75 | - | MS, RI, |
| 78. | Spathulenol | 1587 | 1587 | - | - | 5.34 | - | 0.38 | 0.65 | MS, RI, |
| 79. | Globulol | 1590 | 1590 | - | 1.06 | 3.25 | - | 0.18 | - | MS, RI, |
| 80. | Caryophyllene oxide | 1594 | 1594 | 13.23 | tr | 5.69 | - | 2.14 | MS, RI, AU | |
| 81. | Guaiol | 1602 | 1602 | - | - | - | - | - | 0.53 | MS, RI, |
| 82. | Cubenol | 1606 | 1605 | - | tr | - | - | 1.13 | - | MS, RI, |
| 83. | β-Eudesmol | 1612 | 1613 | - | - | - | - | 0.20 | - | MS, RI, |
| 84. | τ-Eudesmol | 1631 | 1631 | - | - | 0.66 | - | - | MS, RI, | |
| 85. | τ-Muurolol | 1652 | 1652 | 2.03 | - | - | tr | 3.49 | 1.02 | MS, RI, |
| 86. | δ-Cadinol | 1656 | 1656 | tr | 2.82 | - | tr | 0.79 | - | MS, RI, |
| 87. | τ-Muurolol | 1665 | 1661 | 4.64 | - | - | - | - | MS, RI, | |
| 88. | α-Eudesmol | 1666 | 1662 | - | 2.54 | - | - | 1.01 | MS, RI, | |
| 89. | α-Cadinol | 1669 | 1669 | - | - | - | - | 8.14 | - | MS, RI, |
| 90. | Cedr-8-en-13-ol | 1682 | 1688 | 2.17 | - | - | - | - | MS, RI, | |
| 91. | α-Bisabolol | 1692 | 1692 | - | - | - | - | 0.56 | - | MS, RI, |
| 92. | Farnesol | 1726 | 1725 | 0.82 | - | - | - | 1.13 | - | MS, RI, |
| 93. | Hexadecanal | 1817 | 1819 | - | - | - | - | - | 1.16 | MS, RI, |
| 94. | Hexahydrofarnesyl acetone | 1845 | 1845 | - | tr | - | - | - | - | MS, RI, |
| 95. | Palmitic acid | 1977 | 1975 | - | - | - | 39.03 | - | - | MS, RI, AU |
| 96. | trans-9-Octadecen-1-ol | 2068 | 2068 | - | - | 1.31 | - | - | - | MS, RI, |
| 97. | Heptadecanoic acid ethyl ester | 2082 | 2082 | 1.06 | - | - | - | - | - | MS, RI, |
| 98. | trans-Phytol | 2120 | 2122 | 2.69 | - | 0.42 | - | - | - | MS, RI, |
| 99. | Docosane | 2200 | 2200 | - | - | 0.60 | 1.47 | - | - | MS, RI, |
| 100. | Tricosane | 2301 | 2300 | - | - | 0.34 | tr | - | 2.66 | MS, RI, |
| 101. | Tetracosane | 2395 | 2400 | - | - | 0.51 | 4.49 | - | - | MS, RI, |
| 102. | 10,13 Docosadienoic acid methyl ester | 2449 | 2449 | 15.2 | - | - | - | - | 69.61 | MS, RI, |
| 103. | Pentacosane | 2498 | 2500 | 0.66 | - | 0.95 | 6.26 | - | - | MS, RI, |
| 104. | Hexacosane | 2598 | 2600 | 0.63 | - | 1.10 | 8.99 | - | 3.23 | MS, RI, |
| 105. | Heptacosane | 2697 | 2700 | 1.26 | - | 1.35 | 10.27 | - | - | MS, RI, |
| 106. | Octacosane | 2790 | 2800 | 0.77 | - | 1.13 | 9.60 | - | tr | MS, RI, |
| Monoterpene hydrocarbons | 24.9 | 42.91 | 26.27 | 13.40 | 61.95 | - | ||||
| Oxygenated monoterpene | tr | 34.82 | 35.60 | - | 5.69 | 0.49 | ||||
| Sesquiterpene hydrocarbons | 18.97 | 5.69 | 2.05 | tr | 6.37 | 3.80 | ||||
| Oxygenated sesquiterpene | 24.59 | 10.9 | 15.52 | tr | 18.49 | 5.35 | ||||
| Others | 23.64 | 2.11 | 7.87 | 82.55 | 0.40 | 79.84 | ||||
| Total | 92.10 | 96.43 | 87.31 | 95.95 | 92.90 | 89.48 | ||||
| Fc | Fk | Fp | Fs | Ft | Fv | |
|---|---|---|---|---|---|---|
| Fc | - | 0.58 *** | 0.35 *** | −0.02 | 0.71 *** | 0.47 *** |
| Fk | 0.58 *** | - | 0.43 *** | −0.02 | 0.89 *** | −0.03 |
| Fp | 0.35 *** | 0.43 *** | - | 0.05 | 0.45 *** | −0.03 |
| Fs | −0.02 | −0.02 | 0.05 | - | −0.002 | −0.02 |
| Ft | 0.71 *** | 0.89 *** | 0.45 *** | −0.002 | - | −0.03 |
| Fv | 0.47 *** | −0.03 | −0.03 | −0.02 | −0.03 | - |
| Samples | ABTS (mgTE/g Oil) | CUPRAC (mgTE/g Oil) | FRAP (mgTE/g Oil) | PM (mmolTE/g Oil) |
|---|---|---|---|---|
| F. caratavica (Fc) | 41.36 ± 1.27 a | 83.54 ± 3.13 c | 47.34 ± 0.65 e | 5.59 ± 0.01 f |
| F. kuchistanica (Fk) | 29.12 ± 0.85 b | 120.43 ± 9.36 b | 80.74 ± 0.25 c | 36.42 ± 0.07 c |
| F. pseudoreoselinum (Fp) | 22.68 ± 1.03 c | 289.45 ± 7.30 a | 121.64 ± 0.01 b | 50.86 ± 0.07 b |
| F. samarcandica (Fs) | 11.84 ± 1.37 d | 74.39 ± 4.73 c,d | 43.21 ± 0.48 f | 14.37 ± 0.04 e |
| F. tenuisecta (Ft) | 28.03 ± 3.89 b | 278.87 ± 8.51 a | 136.81 ± 1.98 a | 78.66 ± 0.15 a |
| F. varia (Fv) | 7.04 ± 0.47 e | 65.90 ± 1.66 d | 55.00 ± 0.18 d | 15.33 ± 0.07 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, F.S.; Mamatkhanova, M.A.; Mamadalieva, N.Z.; Zengin, G.; Aripova, S.F.; Alshammari, E.; Ashour, M.L. Chemical Profiling and Discrimination of Essential Oils from Six Ferula Species Using GC Analyses Coupled with Chemometrics and Evaluation of Their Antioxidant and Enzyme Inhibitory Potential. Antibiotics 2020, 9, 518. https://doi.org/10.3390/antibiotics9080518
Youssef FS, Mamatkhanova MA, Mamadalieva NZ, Zengin G, Aripova SF, Alshammari E, Ashour ML. Chemical Profiling and Discrimination of Essential Oils from Six Ferula Species Using GC Analyses Coupled with Chemometrics and Evaluation of Their Antioxidant and Enzyme Inhibitory Potential. Antibiotics. 2020; 9(8):518. https://doi.org/10.3390/antibiotics9080518
Chicago/Turabian StyleYoussef, Fadia S., Munira A. Mamatkhanova, Nilufar Z. Mamadalieva, Gokhan Zengin, Salima F. Aripova, Elham Alshammari, and Mohamed L. Ashour. 2020. "Chemical Profiling and Discrimination of Essential Oils from Six Ferula Species Using GC Analyses Coupled with Chemometrics and Evaluation of Their Antioxidant and Enzyme Inhibitory Potential" Antibiotics 9, no. 8: 518. https://doi.org/10.3390/antibiotics9080518
APA StyleYoussef, F. S., Mamatkhanova, M. A., Mamadalieva, N. Z., Zengin, G., Aripova, S. F., Alshammari, E., & Ashour, M. L. (2020). Chemical Profiling and Discrimination of Essential Oils from Six Ferula Species Using GC Analyses Coupled with Chemometrics and Evaluation of Their Antioxidant and Enzyme Inhibitory Potential. Antibiotics, 9(8), 518. https://doi.org/10.3390/antibiotics9080518