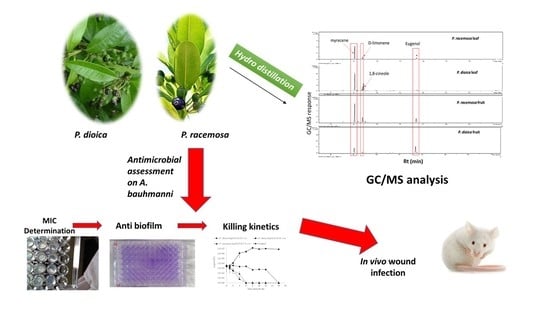
Pimenta Oil as a Potential Treatment for Acinetobacter baumannii Wound Infection: In Vitro and In Vivo Bioassays in Relation to Its Chemical Composition
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Essential Oil
3.3. GC/MS Analysis of the Volatile Oil Composition
3.4. Bacterial Isolates and Culture Conditions
3.5. Determination of the Minimum Inhibitory Concentration (MIC) by Agar Microdilution Technique
3.6. Effect of Essential Oils on Biofilm Formation and Eradication
3.6.1. Biofilm Inhibition Assay
3.6.2. Biofilm Eradication Assay
3.7. Determination of the Minimum Bactericidal Concentration (MBC) by Broth Microdilution Technique
3.8. Kill Kinetics Assay
3.9. In Vivo Wound Infection Animal Model
3.9.1. Ethical Statement
3.9.2. Experimental Design and Induction of Infection
- Group 1: P. dioica leaf E.O. dissolved in sweet almond oil (5.2 µg·mL−1).
- Group 2: P. racemosa leaf E.O. dissolved in sweet almond oil (5.2 µg·mL−1).
- Group 3: Eugenol dissolved in sweet almond oil (2 µg·mL−1)
- Group 4: Cefepime solution (25 µg per gram of mouse weight).
- Group 5: Vehicle (sweet almond oil).
- Group 6: Phosphate-buffered saline.
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Mihu, M.R.; Sandkovsky, U.; Han, G.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence 2010, 1, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [Green Version]
- Bassetti, M.; Ginocchio, F.; Mikulska, M. New treatment options against gram-negative organisms. Crit. Care 2011, 15, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Parekh, J.; Jadeja, D.; Chanda, S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 2006, 29, 203–210. [Google Scholar]
- Morton, J.F. Atlas of Medicinal Plants of Middle America: Bahamas to Yucatan; Charles C. Thomas: Springfield, IL, USA, 1981. [Google Scholar]
- Chaverri, C.; Cicció, J.F. Leaf and fruit essential oil compositions of Pimenta guatemalensis (Myrtaceae) from Costa Rica. Rev. De Biol. Trop. 2015, 63, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Moreno, B.Z. Chemical Composition of Essential Oil of Genus Pimenta (Myrtaceae). In Potential of Essential Oils; El-Shemy, H., Ed.; IntechOpen Limited: London, UK, 2018; p. 21. [Google Scholar] [CrossRef] [Green Version]
- Seidemann, J. World Spice Plants: Economic Usage, Botany, Taxonomy; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006, 73, 236–244. [Google Scholar] [CrossRef]
- Tucker, A.O.; Maciarello, M.J.; Adams, R.P.; Landrum, L.R.; Zanoni, T.A. Volatile leaf oils of Caribbean Myrtaceae. I. Three varieties of Pimenta racemosa (Miller) J. Moore of the Dominican Republic and the commercial bay oil. J. Essent. Oil Res. 1991, 3, 323–329. [Google Scholar] [CrossRef]
- Ayedoun, A.M.; Adeoti, B.S.; Setondji, J.; Menut, C.; Lamaty, G.; Bessiére, J.-M. Aromatic plants from Tropical West Africa. IV. Chemical composition of leaf oil of Pimenta racemosa (Miller) JW Moore var. racemosa from Benin. J. Essent. Oil Res. 1996, 8, 207–209. [Google Scholar] [CrossRef]
- Alitonou, G.A.; Noudogbessi, J.-P.; Sessou, P.; Tonouhewa, A.; Avlessi, F.; Menut, C.; Sohounhloue, D. Chemical composition and biological activities of essential oils of Pimenta racemosa (Mill.) JW Moore. from Benin. Int. J. Biosci. 2012, 2, 1–12. [Google Scholar]
- Tucker, A.O.; Maciarello, M.J.; Landrum, L.R. Volatile leaf oils of Caribbean Myrtaceae. II. Pimenta dioica (L.) Merr. of Jamaica. J. Essent. Oil Res. 1991, 3, 195–196. [Google Scholar] [CrossRef]
- Prasad, M.A.; Zolnik, C.P.; Molina, J.J. Leveraging phytochemicals: The plant phylogeny predicts sources of novel antibacterial compounds. Future Sci. OA 2019, 5, FSO407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Sánchez, D.; Galvão, J.A.; Ambrosio, C.M.; Gloria, E.M.; Oetterer, M. Single and binary applications of essential oils effectively control Listeria monocytogenes biofilms. Ind. Crop. Prod. 2018, 121, 452–460. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Galvão, J.A.; Mazine, M.R.; Gloria, E.M.; Oetterer, M. Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int. J. Food Microbiol. 2018, 286, 128–138. [Google Scholar] [CrossRef]
- Yadav, M.K.; Chae, S.-W.; Im, G.J.; Chung, J.-W.; Song, J.-J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef] [Green Version]
- Shehabeldine, A.M.; Ashour, R.M.; Okba, M.M.; Saber, F.R. Callistemon citrinus bioactive metabolites as new inhibitors of methicillin-resistant Staphylococcus aureus biofilm formation. J. Ethnopharmacol. 2020, 254, 112669. [Google Scholar] [CrossRef]
- Vasavi, H.S.; Arun, A.B.; Rekha, P.D. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pac. J. Trop. Biomed. 2013, 3, 954–959. [Google Scholar] [CrossRef] [Green Version]
- Hernández Díaz, L.; Rodríguez Jorge, M.; García, D.; Pino Alea, J. Actividad antidermatofítica in vitro de aceites esenciales. Rev. Cuba. Plantas Med. 2003, 8. [Google Scholar]
- Goldbeck, J.C.; do Nascimento, J.E.; Jacob, R.G.; Fiorentini, Â.M.; da Silva, W.P. Bioactivity of essential oils from Eucalyptus globulus and Eucalyptus urograndis against planktonic cells and biofilms of Streptococcus mutans. Ind. Crop. Prod. 2014, 60, 304–309. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1, 8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Rao, V. 1, 8-cineol, a food flavoring agent, prevents ethanol-induced gastric injury in rats. Digest. Dis. Sci. 2001, 46, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ćirić, A.; Ristić, M.; Grubišić, D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157: H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef] [Green Version]
- Dhara, L.; Tripathi, A. Antimicrobial activity of eugenol and cinnamaldehyde against extended spectrum beta lactamase producing enterobacteriaceae by in vitro and molecular docking analysis. Eur. J. Integr. Med. 2013, 5, 527–536. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Cunha, B.A. Optimal therapy for multidrug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 2010, 16, 170–171. [Google Scholar] [CrossRef]
- Devi, K.P.; Sakthivel, R.; Nisha, S.A.; Suganthy, N.; Pandian, S.K. Eugenol alters the integrity of cell membrane and acts against the nosocomial pathogen Proteus mirabilis. Arch. Pharmacal Res. 2013, 36, 282–292. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiolology 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Niu, H.; Zhang, W.; Mu, H.; Sun, C.; Duan, J. Synergy among thymol, eugenol, berberine, cinnamaldehyde and streptomycin against planktonic and biofilm-associated food-borne pathogens. Lett. Appl. Microbiolol. 2015, 60, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.R.; de Araújo, A.C.J.; dos Santos Barbosa, C.R.; Muniz, D.F.; da Silva, A.C.A.; Rocha, J.E.; de Morais Oliveira-Tintino, C.D.; Ribeiro-Filho, J.; da Silva, L.E.; Confortin, C. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. and α-pinene. Ind. Crop. Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Seifert, H.; Dijkshoorn, L.; Gerner-Smidt, P.; Pelzer, N.; Tjernberg, I.; Vaneechoutte, M. Distribution of Acinetobacter species on human skin: Comparison of phenotypic and genotypic identification methods. J. Clin. Microbiol. 1997, 35, 2819–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldoghaim, F.S.; Flematti, G.R.; Hammer, K.A. Antimicrobial activity of several cineole-rich Western Australian Eucalyptus essential oils. Microorganisms 2018, 6, 122. [Google Scholar] [CrossRef] [Green Version]
- Karumathil, D.P.; Surendran-Nair, M.; Venkitanarayanan, K. Efficacy of Trans-cinnamaldehyde and Eugenol in Reducing Acinetobacter baumannii Adhesion to and Invasion of Human Keratinocytes and Controlling Wound Infection In Vitro. Phytother. Res. 2016, 30, 2053–2059. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Lin, C.-S.; Hsu, C.-R.; Chang, C.-M.; Chang, I.-W.; Lin, L.-W.; Hung, C.-H.; Wang, J.-L. Using the Chinese herb Scutellaria barbata against extensively drug-resistant Acinetobacter baumannii infections: In vitro and in vivo studies. Bmc Complementary Altern. Med. 2018, 18, 96. [Google Scholar] [CrossRef]
- Egyptian Pharmacopeia. Central Administration of Pharmaceutical Affairs (CAPA), 4th ed.; Ministry of Health and Population: Cairo, Egypt, 2005; pp. 31–33. [Google Scholar]
- Farag, M.A.; Wessjohann, L.A. Volatiles profiling in medicinal licorice roots using steam distillation and solid-phase microextraction (SPME) coupled to chemometrics. J. Food Sci. 2012, 77, C1179–C1184. [Google Scholar] [CrossRef]
- Kashif, M.T.; Yassin, A.S.; Hosny, A.E.-D.M. Detection of AmpC beta-lactamases using sodium salicylate. J. Microbiol. Methods 2012, 91, 354–357. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The agar microdilution method–a new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Naves, P.; Del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Rodríguez-Cerrato, V.; Ponte, M.; Soriano, F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J. Appl. Microbiol. 2008, 105, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.H.; Samir, R.; Aziz, R.K.; Toama, M.A. Two putative MmpL homologs contribute to antimicrobial resistance and nephropathy of enterohemorrhagic E. coli O157: H7. Gut Pathog. 2019, 11, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratton, C.; Weeks, L.; Aldridge, K. Comparison of kill-kinetic studies with agar and broth microdilution methods for determination of antimicrobial activity of selected agents against members of the Bacteroides fragilis group. J. Clin. Microbiol. 1987, 25, 645–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ghasri, P.; Amir, M.; Hwang, B.; Hou, Y.; Khilili, M.; Lin, A.; Keene, D.; Uitto, J.; Woodley, D.T. Topical application of recombinant type VII collagen incorporates into the dermal–epidermal junction and promotes wound closure. Mol. Ther. 2013, 21, 1335–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Isolate | P. dioica Leaf a | P. racemosa Leaf a | P. dioica Berry a | P. racemosa Berry a | Eugenol a | Cefepime b |
|---|---|---|---|---|---|---|
| AB-1 | 0.69 ± 0.2 | 0.52 ± 0.0 | 0.69 ± 0.2 | 0.69 ± 0.2 | 0.10 ± 0.0 | 10 ± 0.0 |
| AB-2 | 0.86 ± 0.2 | 4.84 ± 0.5 | 5.18 ± 0.0 | 0.69 ± 0.2 | 0.20 ± 0.0 | 5.0 ± 0.0 |
| AB-3 | 5.18 ± 0.0 | 1.03 ± 0.0 | 1.03 ± 0.0 | 1.03 ± 0.0 | 0.53 ± 0.0 | 0.1 ± 0.0 |
| AB-5 | 0.86 ± 0.2 | 0.86 ± 0.2 | 0.86 ± 0.2 | 0.86 ± 0.2 | 0.21 ± 0.0 | 5.0± 0.0 |
| AB-6 | 0.69 ± 0.2 | 0.52 ± 0.0 | 0.69 ± 0.2 | 0.69 ± 0.2 | 0.53 ± 0.0 | 0.6 ± 0.0 |
| AB-8 | 0.52 ± 0.0 | 0.52 ± 0.0 | 5.18 ± 0.0 | 0.69 ± 0.2 | 0.21 ± 0.0 | 6.7 ± 2.8 |
| AB-9 | 0.52 ± 0.0 | 0.52 ± 0.0 | 4.49 ± 1.1 | 1.03 ± 0.0 | 0.53±0.0 | 5.0 ± 0.0 |
| AB-10 | 5.18 ± 0.0 | 0.69 ± 0.2 | 5.18 ± 0.0 | 0.69 ± 0.2 | 0.53 ± 0.0 | 0.1 ± 0.0 |
| AB-13 | 1.03 ± 0.0 | 0.52 ± 0.0 | 0.52 ± 0.0 | 1.03 ± 0.0 | 0.45 ± 0.0 | 1.3 ± 0.0 |
| AB-15 | 1.03 ± 0.0 | 1.03 ± 0.0 | 5.18 ± 0.0 | 1.03 ± 0.0 | 5.3 ± 0.0 | 5.0 ± 0.0 |
| AB-11Q | 0.86 ± 0.2 | 1.03 ± 0.0 | 5.18 ± 0.0 | 1.03 ± 0.0 | 0.53 ± 0.0 | 0.1 ± 0.0 |
| AB-14T | 1.03 ± 0.0 | 4.66 ± 0.8 | 5.18 ± 0.0 | 5.18 ± 0.0 | 0.78 ± 0.2 | 0.1 ± 0.0 |
| AB-16Q | 1.03 ± 0.0 | 1.03 ± 0.0 | 5.18 ± 0.0 | 5.87 ± 1.1 | 0.88 ± 0.2 | 0.3 ± 0.0 |
| AB-7T | 4.49 ± 1.1 | 5.18 ± 0.0 | 5.18 ± 0.0 | 5.18 ± 0.0 | 0.53 ± 0.0 | 1.0 ± 0.30 |
| ATCC 19606 | 0.69 ± 0.2 | 1.03 ± 0.0 | 0.86 ± 0.2 | 1.03 ± 0.0 | 0.47 ± 0.0 | 0.1 ± 0.0 |
| Average MIC values (µg·mL−1) | 1.65 | 1.60 | 3.38 | 1.78 | 0.79 | 2.70 |
| Essential Oils/Standard Compound | MBC Value | MIC Values Equivalents |
|---|---|---|
| P. dioicaleaf | 1.0 µg·mL−1 | 2× MIC |
| P. racemosaleaf | 2.0 µg·mL−1 | 4× MIC |
| P. racemosaberry | 2.76 µg·mL−1 | 4× MIC |
| P. dioicaberry * | More than 41.4 µg·mL−1 | More than 8× MIC |
| Eugenol | 1.2 µg·mL−1 | 6× MIC |
| RI * Calculated. | RI Reported | Name | Class | Essential Oil | |||
|---|---|---|---|---|---|---|---|
| P. racemosa Leaf | P. dioica Leaf | P. racemosa Berry | P. dioica Berry | ||||
| 1186 | 1201 | Decanal | aldehyde/ketone | 0.0 | 0.03 | 0.0 | 0.0 |
| Total aldehyde/ketone | 0.0 | 0.03 | 0.0 | 0.0 | |||
| 906 | 932 | α-Pinene | 1.3 | 1.0 | 1.5 | 0.2 | |
| 965.2 | 988 | β-Myrcene | 39.6 | 44.1 | 42.3 | 13.9 | |
| 978.8 | 1003 | p-Mentha-1(7),8-diene | 0.9 | 0.2 | 2.9 | 0.0 | |
| 980.9 | 1005 | α-Phellandrene | 1.0 | 0.6 | 0.6 | 1.0 | |
| 991.2 | 1014 | α-Terpinene | 0.6 | 0.4 | 0.9 | 0.0 | |
| 993 | 1014 | 4-carene | 0.0 | 2.1 | 0.0 | 0.4 | |
| 1001 | 1020 | p-Cymene | 2.3 | 2.1 | 0.9 | 0.4 | |
| 1004.2 | 1024 | Limonene | 15.5 | 11.7 | 14.3 | 4.6 | |
| 1019 | 1032 | β-cis-Ocimene | 2.8 | 0.6 | 4.6 | 1.1 | |
| 1033 | 1054 | γ-Terpinene | 0.2 | 0.4 | 0.7 | 0.4 | |
| 1063 | 1085 | p-Mentha-2,4(8)-diene | 0.1 | 0.0 | 0.4 | 0.3 | |
| Total Monoterpene hydrocarbons | 64.4 | 63.3 | 69.1 | 22.2 | |||
| 1009.5 | 1026 | 1,8-cineol | Oxygenated monoterpene | 0.0 | 18.8 | 0.0 | 1.5 |
| 1080 | 1095 | β-Linalool | 2.1 | 5.3 | 1.6 | 3.6 | |
| 1163 | 1174 | Terpinen-4-ol | 0.8 | 1.5 | 1.0 | 1.4 | |
| 1181.5 | 1186 | α-Terpineol | 0.3 | 2.5 | 0.0 | 2.2 | |
| 1250 | 1247 | Chavicol | 1.5 | 0.0 | 0.2 | 1.5 | |
| 1250.1 | 1264 | Geranial | 0.0 | 0.0 | 0.0 | 0.6 | |
| Total oxygenated Monoterpene | 4.7 | 28.1 | 2.8 | 10.8 | |||
| 1334.5 | 1356 | Eugenol | Phenols | 31.0 | 8.6 | 27.7 | 65.6 |
| 1350 | 1345 | α-Cubebene | Sesquiterpene hydrocarbon | 0.0 | 0.0 | 0.0 | 0.1 |
| 1349 | 1374 | α-Copaene | 0.0 | 0.0 | 0.3 | 0.0 | |
| 1396 | 1417 | β-Caryophyllene | 0.0 | 0.0 | 0.0 | 0.3 | |
| 1430 | 1452 | α-Humulene | 0.0 | 0.0 | 0.0 | 0.2 | |
| 1444.8 | 1484 | Germacrene D | 0.0 | 0.0 | 0.0 | 0.2 | |
| 1478 | 1522 | δ-Cadinene | 0.0 | 0.0 | 0.0 | 0.6 | |
| Total sesquiterpenes | 0.0 | 0.0 | 0.3 | 1.4 | |||
| Total | 100 | 100 | 100 | 100 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.M.; Samir, R.; Saber, F.R.; Ahmed, S.R.; Farag, M.A. Pimenta Oil as a Potential Treatment for Acinetobacter baumannii Wound Infection: In Vitro and In Vivo Bioassays in Relation to Its Chemical Composition. Antibiotics 2020, 9, 679. https://doi.org/10.3390/antibiotics9100679
Ismail MM, Samir R, Saber FR, Ahmed SR, Farag MA. Pimenta Oil as a Potential Treatment for Acinetobacter baumannii Wound Infection: In Vitro and In Vivo Bioassays in Relation to Its Chemical Composition. Antibiotics. 2020; 9(10):679. https://doi.org/10.3390/antibiotics9100679
Chicago/Turabian StyleIsmail, Maha M., Reham Samir, Fatema R. Saber, Shaimaa R. Ahmed, and Mohamed A. Farag. 2020. "Pimenta Oil as a Potential Treatment for Acinetobacter baumannii Wound Infection: In Vitro and In Vivo Bioassays in Relation to Its Chemical Composition" Antibiotics 9, no. 10: 679. https://doi.org/10.3390/antibiotics9100679
APA StyleIsmail, M. M., Samir, R., Saber, F. R., Ahmed, S. R., & Farag, M. A. (2020). Pimenta Oil as a Potential Treatment for Acinetobacter baumannii Wound Infection: In Vitro and In Vivo Bioassays in Relation to Its Chemical Composition. Antibiotics, 9(10), 679. https://doi.org/10.3390/antibiotics9100679