Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
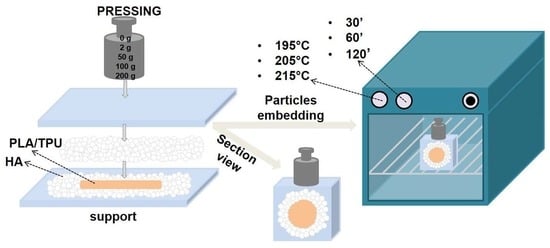
2.1. Core-shell Materials Synthesis
2.2. Characterization Techniques
3. Results and Discussion
3.1. Morpho-compositional Characterization
3.2. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.3. Form Factor Variation
3.4. Mass Variation
3.5. Compression Mechanical Test
3.6. Pull-out Test. Particle Adhesion Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kaya, İ.; Şahin, M.C.; Cingöz, İ.D.; Aydin, N.; Atar, M.; Kizmazoğlu, C.; Kavuncu, S.; Aydin, H.E. Three Dimensional Printing and Biomaterials in the Repairment of Bone Defects; Hydroxyapatite PLA Filaments. Turk. J. Med. Sci. 2019, 49, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Niaza, K.; Senatov, F.; Kaloshkin, S.; Maksimkin, A.; Chukov, D. 3D-printed scaffolds based on PLA/HA nanocomposites for trabecular bone reconstruction. J. Phys. Conf. Ser. 2016, 741, 012068. [Google Scholar] [CrossRef] [Green Version]
- Neacsu, P.; Staras, A.; Voicu, S.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.; Pandele, A.; Croitoru, S.; Miculescu, F. Characterization and in vitro and in vivo assessment of a novel cellulose acetate-coated Mg-based alloy for orthopedic applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [Green Version]
- Ioniță, M.; Crică, L.E.; Voicu, S.I.; Dinescu, S.; Miculescu, F.; Costache, M.; Iovu, H. Synergistic effect of carbon nanotubes and graphene for high performance cellulose acetate membranes in biomedical applications. Carbohydr. Polym. 2018, 183, 50–61. [Google Scholar] [CrossRef]
- Stan, G.; Popa, A.; Bojin, D. Bioreactivity evaluation in simulated body fluid of magnetron sputtered glass and glass-ceramic coatings: A FTIR spectroscopy study. Dig. J. Nanomater. Biostructures 2010, 5, 557–566. [Google Scholar]
- Ionita, M.; Vasile, E.; Crica, L.E.; Voicu, S.I.; Pandele, A.M.; Dinescu, S.; Predoiu, L.; Galateanu, B.; Hermenean, A.; Costache, M. Synthesis, characterization and in vitro studies of polysulfone/graphene oxide composite membranes. Compos. Part B Eng. 2015, 72, 108–115. [Google Scholar] [CrossRef]
- Voicu, S.; Pandele, M.; Vasile, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The impact of sonication time through polysulfone-graphene oxide composite films properties. Dig. J. Nanomater. Biostructures 2013, 8, 1389–1394. [Google Scholar]
- Wu, J.; Chen, N.; Bai, F.; Wang, Q. Preparation of poly (vinyl alcohol)/poly (lactic acid)/hydroxyapatite bioactive nanocomposites for fused deposition modeling. Polym. Compos. 2018, 39, E508–E518. [Google Scholar] [CrossRef]
- Antoniac, I.; Popescu, D.; Zapciu, A.; Antoniac, A.; Miculescu, F.; Moldovan, H. Magnesium Filled Polylactic Acid (PLA) Material for Filament Based 3D Printing. Materials 2019, 12, 719. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B.W.; Murray, J.W.; Grant, D.M. Two step porosification of biomimetic thin-film hydroxyapatite/alpha-tri calcium phosphate coatings by pulsed electron beam irradiation. Sci. Rep. 2018, 8, 14530. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.; Bogdan, I.; Pasuk, I.; Ferreira, J.; Stan, G. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Vladescu, A.; Cotrut, C.M.; Azem, F.A.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef] [Green Version]
- Matos, B.D.M.; Rocha, V.; da Silva, E.J.; Moro, F.H.; Bottene, A.C.; Ribeiro, C.A.; dos Santos Dias, D.; Antonio, S.G.; do Amaral, A.C.; Cruz, S.A. Evaluation of commercially available polylactic acid (PLA) filaments for 3D printing applications. J. Therm. Anal. Calorim. 2019, 137, 555–562. [Google Scholar] [CrossRef]
- Manganiello, C.; Naso, D.; Cupertino, F.; Fiume, O.; Percoco, G. Investigating the Potential of Commercial-Grade Carbon Black-Filled TPU for the 3D Printing of Compressive Sensors. Micromachines 2019, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Haryńska, A.; Gubanska, I.; Kucinska-Lipka, J.; Janik, H. Fabrication and Characterization of Flexible Medical-Grade TPU Filament for Fused Deposition Modeling 3DP Technology. Polymers 2018, 10, 1304. [Google Scholar] [CrossRef] [Green Version]
- Miculescu, F.; Miculescu, M.; Ciocan, L.; Ernuteanu, A.; Antoniac, I.; Pencea, I.; Matei, E. Comparative studies regarding heavy elements concentration in human cortical bone. Dig. J. Nanomater. Biostructures 2011, 6, 1117–1127. [Google Scholar]
- Miculescu, F.; Stan, G.; Ciocan, L.; Miculescu, M.; Berbecaru, A.; Antoniac, I. Cortical bone as resource for producing biomimetic materials for clinical use. Dig. J. Nanomater. Biostructures 2012, 7, 1667–1677. [Google Scholar]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2018, 438, 158–166. [Google Scholar] [CrossRef]
- Cicala, G.; Giordano, D.; Tosto, C.; Filippone, G.; Recca, A.; Blanco, I. Polylactide (PLA) filaments a biobased solution for additive manufacturing: Correlating rheology and thermomechanical properties with printing quality. Materials 2018, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
- Corcione, C.E.; Scalera, F.; Gervaso, F.; Montagna, F.; Sannino, A.; Maffezzoli, A. One-step solvent-free process for the fabrication of high loaded PLA/HA composite filament for 3D printing. J. Therm. Anal. Calorim. 2018, 134, 575–582. [Google Scholar] [CrossRef]
- Popescu, D.; Zapciu, A.; Amza, C.; Baciu, F.; Marinescu, R. FDM process parameters influence over the mechanical properties of polymer specimens: A review. Polym. Test. 2018, 69, 157–166. [Google Scholar] [CrossRef]
- Motaparti, K.P.; Taylor, G.; Leu, M.C.; Chandrashekhara, K.; Castle, J.; Matlack, M. Experimental investigation of effects of build parameters on flexural properties in fused deposition modelling parts. Virtual Phys. Prototyp. 2017, 12, 207–220. [Google Scholar] [CrossRef]
- Zaldivar, R.; Witkin, D.; McLouth, T.; Patel, D.; Schmitt, K.; Nokes, J. Influence of processing and orientation print effects on the mechanical and thermal behavior of 3D-Printed ULTEM® 9085 Material. Addit. Manuf. 2017, 13, 71–80. [Google Scholar] [CrossRef]
- Tanikella, N.G.; Wittbrodt, B.; Pearce, J.M. Tensile strength of commercial polymer materials for fused filament fabrication 3D printing. Addit. Manuf. 2017, 15, 40–47. [Google Scholar] [CrossRef] [Green Version]
- McLouth, T.D.; Severino, J.V.; Adams, P.M.; Patel, D.N.; Zaldivar, R.J. The impact of print orientation and raster pattern on fracture toughness in additively manufactured ABS. Addit. Manuf. 2017, 18, 103–109. [Google Scholar] [CrossRef]
- Yu, L.Y.; Zhu, B.; Cai, X.; Wang, Y.W.; Han, R.H.; Li, Y.W. Review of polymer surface modification method. Mater. Sci. Forum 2016, 852, 626–631. [Google Scholar] [CrossRef]
- Ozeki, K.; Hirakuri, K. The effect of nitrogen and oxygen plasma on the wear properties and adhesion strength of the diamond-like carbon film coated on PTFE. Appl. Surf. Sci. 2008, 254, 1614–1621. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [Green Version]
- Bielawski, M.; Beres, W. FE modelling of surface stresses in erosion-resistant coatings under single particle impact. Wear 2007, 262, 167–175. [Google Scholar] [CrossRef]
- van Kampen, A.; Kohlus, R. Statistical modelling of coating layer thickness distributions: Influence of overspray on coating quality. Powder Technol. 2018, 325, 557–567. [Google Scholar] [CrossRef]
- Bogdanovich, V.; Giorbelidze, M. Mathematical modelling of powder material motion and transportation in high-temperature flow core during plasma coatings application. IOP Conf. Ser. Mater. Sci. Eng. 2018, 327, 022036. [Google Scholar] [CrossRef]
- Dyshlovenko, S.; Pawlowski, L.; Pateyron, B.; Smurov, I.; Harding, J. Modelling of plasma particle interactions and coating growth for plasma spraying of hydroxyapatite. Surf. Coat. Technol. 2006, 200, 3757–3769. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Setiawan, M.; Palaniappan, A.; Yam, G.H.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Functionalization of the polymeric surface with bioceramic nanoparticles via a novel, nonthermal dip coating method. ACS Appl. Mater. Interfaces 2016, 8, 35565–35577. [Google Scholar] [CrossRef]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, Q.; Lin, J.-H.; Lou, C.-W. Properties and mechanism of hydroxyapatite coating prepared by electrodeposition on a braid for biodegradable bone scaffolds. Nanomaterials 2019, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Petrovskaya, T.; Toropkov, N.; Mironov, E.; Azarmi, F. 3D printed biocompatible polylactide-hydroxyapatite based material for bone implants. Mater. Manuf. Process. 2018, 33, 1899–1904. [Google Scholar] [CrossRef]
- Dudin, S.; Cotrut, C.M.; Dinu, M.; Zykova, A.; Parau, A.C.; Yakovin, S.; Vladescu, A. Comparative study of the hydroxyapatite coatings prepared with/without substrate bias. Ceram. Int. 2017, 43, 14968–14975. [Google Scholar] [CrossRef]
- Miculescu, F.; Jepu, I.; Porosnicu, C.; Lungu, C.; Miculescu, M.; Burhala, B. A study on the influence of the primary electron beam on nanodimensional layers analysis. Dig. J. Nanomater. Biostructures 2011, 6, 335–345. [Google Scholar]
- Miculescu, F.; Ciocan, L.; Miculescu, M.; Ernuteanu, A. Effect of heating process on micro structure level of cortical bone prepared for compositional analysis. Dig. J. Nanomater. Biostructures 2011, 6, 225–233. [Google Scholar]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.; Jeyaratnam, N.; Yuvaraj, A. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Varshney, S.K. Polylactides—chemistry, properties and green packaging technology: A review. Int. J. Food Prop. 2011, 14, 37–58. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.-C.; Voicu, S.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-derived biphasic calcium phosphates through increased phosphorus-based reagent amounts for biomedical applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.; Ciocan, L.; Niculescu, M.; Corobea, M.; Rada, M.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Stan, G.; Miculescu, M.; Voicu, S.; Cîmpean, A.; Mitran, V.; Batalu, D. Tuning hydroxyapatite particles’ characteristics for solid freeform fabrication of bone scaffolds. In Advanced Composite Materials; Tiwari, A., Alenezi, M.R., Jun, S.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 321–397. ISBN 978-111-924-286-4. [Google Scholar]
- Stabile, L.; Scungio, M.; Buonanno, G.; Arpino, F.; Ficco, G. Airborne particle emission of a commercial 3D printer: The effect of filament material and printing temperature. Indoor Air 2017, 27, 398–408. [Google Scholar] [CrossRef]
- van Manen, T.; Janbaz, S.; Zadpoor, A.A. Programming 2D/3D shape-shifting with hobbyist 3D printers. Mater. Horiz. 2017, 4, 1064–1069. [Google Scholar] [CrossRef] [Green Version]
- ASTM:C633. Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Liu, D.-M.; Yang, Q.; Troczynski, T. Sol–gel hydroxyapatite coatings on stainless steel substrates. Biomaterials 2002, 23, 691–698. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Zeng, X.; Khor, K.A.; Weng, W.; Sun, D. Evaluation of adhesion strength and toughness of fluoridated hydroxyapatite coatings. Thin Solid Films 2008, 516, 5162–5167. [Google Scholar] [CrossRef]
- Cheng, K.; Ren, C.; Weng, W.; Du, P.; Shen, G.; Han, G.; Zhang, S. Bonding strength of fluoridated hydroxyapatite coatings: A comparative study on pull-out and scratch analysis. Thin Solid Films 2009, 517, 5361–5364. [Google Scholar] [CrossRef]
- Stan, G.; Morosanu, C.; Marcov, D.; Pasuk, I.; Miculescu, F.; Reumont, G. Effect of annealing upon the structure and adhesion properties of sputtered bio-glass/titanium coatings. Appl. Surf. Sci. 2009, 255, 9132–9138. [Google Scholar] [CrossRef]
- Ozeki, K.; Masuzawa, T.; Aoki, H. Fabrication of hydroxyapatite thin films on polyetheretherketone substrates using a sputtering technique. Mater. Sci. Eng. C 2017, 72, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Rosas, E.; Kuramochi, G.; Etcheberry, S.; Olate, S.; Weber, B. Oral Applications of Cyanoacrylate Adhesives: A Literature Review. BioMed Res. Int. 2019, 2019, 8217602. [Google Scholar] [CrossRef]
- Wady, P.; Wasilewski, A.; Brock, L.; Edge, R.; Baidak, A.; McBride, C.; Leay, L.; Griffiths, A.; Vallés, C. Effect of ionising radiation on the mechanical and structural properties of 3D printed plastics. Addit. Manuf. 2020, 31, 100907. [Google Scholar] [CrossRef]
- Polonio-Alcalá, E.; Rabionet, M.; Gallardo, X.; Angelats, D.; Ciurana, J.; Ruiz-Martínez, S.; Puig, T. PLA Electrospun Scaffolds for Three-Dimensional Triple-Negative Breast Cancer Cell Culture. Polymers 2019, 11, 916. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, H.; Cheon, K.-H.; Park, C.; Jang, T.-S.; Kim, H.-E.; Jung, H.-D. Fabrication of poly (lactic acid)/Ti composite scaffolds with enhanced mechanical properties and biocompatibility via fused filament fabrication (FFF)–based 3D printing. Addit. Manuf. 2019, 30, 100883. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zhang, F.; Leng, J.; Pei, S.; Wang, L.; Jia, X.; Cotton, C.; Sun, B.; Chou, T.-W. Microstructural design for enhanced shape memory behavior of 4D printed composites based on carbon nanotube/polylactic acid filament. Compos. Sci. Technol. 2019, 181, 107692. [Google Scholar] [CrossRef]
- Xie, J.; Zhai, X.; Hse, C.; Shupe, T.; Pan, H. Polyols from microwave liquefied bagasse and its application to rigid polyurethane foam. Materials 2015, 8, 8496–8509. [Google Scholar] [CrossRef] [Green Version]
- Kanabenja, W.; Potiyaraj, P. Graphene/Thermoplastic Polyurethane Composites. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2018; Volume 773, pp. 77–81. [Google Scholar]
- Marycz, K.; Marędziak, M.; Grzesiak, J.; Lis, A.; Śmieszek, A. Biphasic polyurethane/polylactide sponges doped with nano-hydroxyapatite (nHAp) combined with human adipose-derived mesenchymal stromal stem cells for regenerative medicine applications. Polymers 2016, 8, 339. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, P.F.; Advincula, R.C. Mechanically robust, ultraelastic hierarchical foam with tunable properties via 3D printing. Adv. Funct. Mater. 2018, 28, 1800631. [Google Scholar] [CrossRef]
- Wu, G.; Liu, S.; Jia, H.; Dai, J. Preparation and properties of heat resistant polylactic acid (PLA)/nano-SiO2 composite filament. J. Wuhan. Univ. Technol. 2016, 31, 164–171. [Google Scholar] [CrossRef]
- Chacón, J.; Caminero, M.A.; García-Plaza, E.; Núñez, P.J. Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Des. 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Cheng, X.; Li, S.; Murr, L.; Zhang, Z.; Hao, Y.; Yang, R.; Medina, F.; Wicker, R. Compression deformation behavior of Ti–6Al–4V alloy with cellular structures fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2012, 16, 153–162. [Google Scholar] [CrossRef]
- Barriuso, S.; Chao, J.; Jiménez, J.A.; García, S.; González-Carrasco, J.L. Fatigue behavior of Ti6Al4V and 316 LVM blasted with ceramic particles of interest for medical devices. J. Mech. Behav. Biomed. Mater. 2014, 30, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Mocanu, A.-C.; Miculescu, M.; Machedon-Pisu, T.; Maidaniuc, A.; Ciocoiu, R.C.; Ioniță, M.; Pasuk, I.; Stan, G.E.; Miculescu, F. Internal and external surface features of newly developed porous ceramics with random interconnected 3D channels by a fibrous sacrificial porogen method. Appl. Surf. Sci. 2019, 489, 226–238. [Google Scholar] [CrossRef]
- Beltrán-Fernández, J.A.; Hernandez-Gomez, L.H.; Rodriguez-Cañizo, R.G.; Urriolagoitia-Calderón, G.; Urriolagoitia-Sosa, G.; González-Rebatú, A.; Dufoo-Olvera, M. Mechanical behavior of a calcium phosphate ceramic bone graft used in the rehabilitation of a C4 human vertebra. Appl. Mech. Mater. 2007, 7, 101–106. [Google Scholar] [CrossRef]
- Pastor-Artigues, M.-M.; Roure-Fernández, F.; Ayneto-Gubert, X.; Bonada-Bo, J.; Pérez-Guindal, E.; Buj-Corral, I. Elastic Asymmetry of PLA Material in FDM-Printed Parts: Considerations Concerning Experimental Characterisation for Use in Numerical Simulations. Materials 2020, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- He, J.Y.; Ding, Y.M.; Xue, Z.C.; Yang, W.M. Research on the Elasticity Modulus of Short Glass Fiber/Thermoplastic Polyurethane (SGF-TPU) Composites. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; Volume 561, pp. 152–157. [Google Scholar]
- Chen, C.-C.; Chueh, J.-Y.; Tseng, H.; Huang, H.-M.; Lee, S.-Y. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Tripodo, G.; Wischke, C.; Lendlein, A. Highly Flexible Poly (ethyl-2-cyanoacrylate) Based Materials Obtained by Incorporation of Oligo (ethylene glycol) diglycidylether. Macromol. Symp. 2011, 309, 49–58. [Google Scholar] [CrossRef]










| Sample Type | Pressing Force [MPa] | Thermal Treatment | |
|---|---|---|---|
| Exposure Time [min]* | Temperature [°C]* | ||
| Reference | 0 | 30 60 120 | 195 205 215 |
| 0.5 × 10−4 MPa | 0.5 × 10−4 | ||
| 1.5 × 10−4 MPa | 1.5 × 10−4 | ||
| 2.5 × 10−4 MPa | 2.5 × 10−4 | ||
| 5 × 10−4 MPa | 5 × 10−4 | ||
| Sample | Compression Strength [MPa] |
|---|---|
| PLA (obtained at 205 °C temperature, 60’ exposure time of 60’ and 0.5 × 10−4 MPa pressing force) | 27.67 ± 3.02 |
| TPU (obtained at 215 °C temperature, 60’ exposure time of 60’ and 2.5 × 10−4 MPa pressing force) | 42.32 ± 2.73 |
| Longitudinal cortical bone | 115 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dascalu, C.-A.; Miculescu, F.; Mocanu, A.-C.; Constantinescu, A.E.; Butte, T.M.; Pandele, A.M.; Ciocoiu, R.-C.; Voicu, S.I.; Ciocan, L.T. Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications. Coatings 2020, 10, 283. https://doi.org/10.3390/coatings10030283
Dascalu C-A, Miculescu F, Mocanu A-C, Constantinescu AE, Butte TM, Pandele AM, Ciocoiu R-C, Voicu SI, Ciocan LT. Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications. Coatings. 2020; 10(3):283. https://doi.org/10.3390/coatings10030283
Chicago/Turabian StyleDascalu, Catalina-Andreea, Florin Miculescu, Aura-Catalina Mocanu, Andreea Elena Constantinescu, Tudor Mihai Butte, Andreea Madalina Pandele, Robert-Catalin Ciocoiu, Stefan Ioan Voicu, and Lucian Toma Ciocan. 2020. "Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications" Coatings 10, no. 3: 283. https://doi.org/10.3390/coatings10030283
APA StyleDascalu, C. -A., Miculescu, F., Mocanu, A. -C., Constantinescu, A. E., Butte, T. M., Pandele, A. M., Ciocoiu, R. -C., Voicu, S. I., & Ciocan, L. T. (2020). Novel Synthesis of Core-Shell Biomaterials from Polymeric Filaments with a Bioceramic Coating for Biomedical Applications. Coatings, 10(3), 283. https://doi.org/10.3390/coatings10030283