Antimicrobial Peptides-Coated Stainless Steel for Fighting Biofilms Formation for Food and Medical Fields: Review of Literature
Abstract
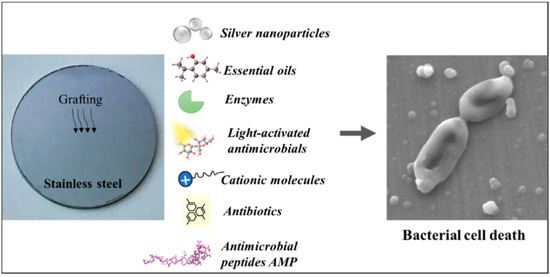
:1. Introduction
2. The Employment of Stainless Steel
3. Antimicrobial Coatings on Stainless Steel Incorporating Biocides
3.1. Silver Nanoparticles Coated on Stainless Steel
3.2. Essential Oils Coated on Stainless Steel
3.3. Light-Activated Antimicrobials Coated on Stainless Steel
3.4. Cationic Molecules Coated on Stainless Steel
3.5. Antibiotics Coated on Stainless Steel
3.6. Enzymes Coated on Stainless Steel
4. Generalities on Antimicrobial Peptides
5. Stainless-Steel Coatings Incorporating Antimicrobial Peptides
6. Nisin Qualification
7. Nisin Antimicrobial Properties and Mechanism of Action
8. Nisin Antimicrobial Spectrum and Applications
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial Biofilms in Nature and Disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Pereira, M.O.; Machado, I.; Simões, L.C.; Vieira, M.J. Comparative antibacterial potential of selected aldehyde-based biocides and surfactants against planktonic Pseudomonas fluorescens. J. Ind. Microbiol. Biotechnol. 2006, 33, 741–749. [Google Scholar] [CrossRef] [Green Version]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Marino, M.; Frigo, F.; Bartolomeoli, I.; Maifreni, M. Safety-Related Properties of Staphylococci Isolated from Food and Food Environments: Safety-Related Properties of Staphylococci. J. Appl. Microbiol. 2011, 110, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Beaupré, G.S.; Csongradi, J.J. Refracture Risk After Plate Removal in the Forearm. J. Orthop. Trauma 1996, 10, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Héquet, A.; Humblot, V.; Berjeaud, J.-M.; Pradier, C.-M. Optimized Grafting of Antimicrobial Peptides on Stainless Steel Surface and Biofilm Resistance Tests. Colloids Surf. B Biointerfaces 2011, 84, 301–309. [Google Scholar] [CrossRef]
- Boyd, A.H.; Hylwa, S.A. Nickel Release from Surgical Instruments and Operating Room Equipment. Dermatol. Online J. 2018, 24. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface Modifications for Antimicrobial Effects in the Healthcare Setting: A Critical Overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Cowan, M.M.; Abshire, K.Z.; Houk, S.L.; Evans, S.M. Antimicrobial Efficacy of a Silver-Zeolite Matrix Coating on Stainless Steel. J. Ind. Microbiol. Biotechnol. 2003, 30, 102–106. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Subbiahdoss, G.; Zeng, G.; Salmi, Z.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Antibacterial Isoeugenol Coating on Stainless Steel and Polyethylene Surfaces Prevents Biofilm Growth. J. Appl. Microbiol. 2018, 124, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic Activity, Antibacterial Effect, and Photoinduced Hydrophilicity of TiO 2 Films Coated on a Stainless Steel Substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Dickinson, G.H.; Teo, S.L.-M.; Rittschof, D. Biomimetic Anchors for Antifouling and Antibacterial Polymer Brushes on Stainless Steel. Langmuir 2011, 27, 7065–7076. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.J.; Denis-Rohr, A.; Goddard, J.M. Antimicrobial Food Equipment Coatings: Applications and Challenges. Annu. Rev. Food Sci. Technol. 2015, 6, 97–118. [Google Scholar] [CrossRef]
- Caro, A.; Humblot, V.; Méthivier, C.; Minier, M.; Salmain, M.; Pradier, C.-M. Grafting of Lysozyme and/or Poly (Ethylene Glycol) to Prevent Biofilm Growth on Stainless Steel Surfaces. J. Phys. Chem. B 2009, 113, 2101–2109. [Google Scholar] [CrossRef]
- Riool, M.; de Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial Peptides in Biomedical Device Manufacturing. Front. Chem. 2017, 5, 63. [Google Scholar] [CrossRef]
- Andrade, C.A. Chemical Immobilization of Antimicrobial Peptides on Biomaterial Surfaces. Front. Biosci. 2016, 8, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Amso, Z.; Hayouka, Z. Antimicrobial Random Peptide Cocktails: A New Approach to Fight Pathogenic Bacteria. Chem. Commun. 2019, 55, 2007–2014. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [Green Version]
- Rathinakumar, R.; Walkenhorst, W.F.; Wimley, W.C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131, 7609–7617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natrajan, N.; Sheldon, B.W. Efficacy of Nisin-Coated Polymer Films to Inactivate Salmonella Typhimurium on Fresh Broiler Skin†. J. Food Prot. 2000, 63, 1189–1196. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical Applications of Nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [Green Version]
- Deshwal, G.K.; Panjagari, N.R. Review on Metal Packaging: Materials, Forms, Food Applications, Safety and Recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Resnik, M.; Benčina, M.; Levičnik, E.; Rawat, N.; Iglič, A.; Junkar, I. Strategies for Improving Antimicrobial Properties of Stainless Steel. Materials 2020, 13, 2944. [Google Scholar] [CrossRef]
- Jullien, C.; Bénézech, T.; Carpentier, B.; Lebret, V.; Faille, C. Identification of Surface Characteristics Relevant to the Hygienic Status of Stainless Steel for the Food Industry. J. Food Eng. 2003, 56, 77–87. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhiman, N.K.; Agnihotri, S.; Shukla, R. Silver-Based Polymeric Nanocomposites as Antimicrobial Coatings for Biomedical Applications. In Nanotechnology in Modern Animal Biotechnology; Singh, S., Maurya, P.K., Eds.; Springer: Singapore, 2019; ISBN 9789811360039. [Google Scholar]
- Falentin-Daudré, C.; Faure, E.; Svaldo-Lanero, T.; Farina, F.; Jérôme, C.; Van De Weerdt, C.; Martial, J.; Duwez, A.-S.; Detrembleur, C. Antibacterial Polyelectrolyte Micelles for Coating Stainless Steel. Langmuir 2012, 28, 7233–7241. [Google Scholar] [CrossRef]
- Zhu, X.; Jun Loh, X. Layer-by-Layer Assemblies for Antibacterial Applications. Biomater. Sci. 2015, 3, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- DeVasConCellos, P.; Bose, S.; Beyenal, H.; Bandyopadhyay, A.; Zirkle, L.G. Antimicrobial Particulate Silver Coatings on Stainless Steel Implants for Fracture Management. Mater. Sci. Eng. C 2012, 32, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Pishbin, F.; Mouriño, V.; Gilchrist, J.B.; McComb, D.W.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Single-Step Electrochemical Deposition of Antimicrobial Orthopaedic Coatings Based on a Bioactive Glass/Chitosan/Nano-Silver Composite System. Acta Biomater. 2013, 9, 7469–7479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-Enhanced Deposition of Silver Nanoparticles onto Polymer and Metal Surfaces for the Generation of Antimicrobial Characteristics. J. Appl. Polym. Sci. 2004, 93, 1411–1422. [Google Scholar] [CrossRef]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the Toxic Effect of Silver Nanoparticles on Mammalian Cell Lines. J. Nanomater. 2015, 2015, 136765. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Yoon, J.U.; Kim, D.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; Chung, Y.H.; Kim, J.; et al. Lung Function Changes in Sprague-Dawley Rats After Prolonged Inhalation Exposure to Silver Nanoparticles. Inhal. Toxicol. 2008, 20, 567–574. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Mayaud, L.; Carricajo, A.; Zhiri, A.; Aubert, G. Comparison of Bacteriostatic and Bactericidal Activity of 13 Essential Oils against Strains with Varying Sensitivity to Antibiotics. Lett. Appl. Microbiol. 2008, 47, 167–173. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Cazzola, M.; Ferraris, S.; Allizond, V.; Banche, G.; Bertea, C.; di Confiengo, G.; Novara, C.; Cochis, A.; Rimondini, L.; Spriano, S. Surface Coating and Functionalization of Metallic Biomaterials with Essential Oils for Antibacterial Applications. In Proceedings of the Proceedings of 1st Coatings and Interfaces; MDPI: Basel, Switzerland, 2019; p. 6156. [Google Scholar]
- Franklyne, J.S.; Mukherjee, A.; Chandrasekaran, N. Essential Oil Micro- and Nanoemulsions: Promising Roles in Antimicrobial Therapy Targeting Human Pathogens. Lett. Appl. Microbiol. 2016, 63, 322–334. [Google Scholar] [CrossRef]
- Akram, M.Z.; Fırıncıoğlu, S.Y.; Jalal, H.; Doğan, S.C. The Use of Essential Oils in Active Food Packaging: A Review of Recent Studies. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 1799–1804. [Google Scholar] [CrossRef] [Green Version]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Huang, Z. A Review of Progress in Clinical Photodynamic Therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Noimark, S.; Bovis, M.; MacRobert, A.J.; Correia, A.; Allan, E.; Wilson, M.; Parkin, I.P. Photobactericidal Polymers; the Incorporation of Crystal Violet and Nanogold into Medical Grade Silicone. RSC Adv. 2013, 3, 18383. [Google Scholar] [CrossRef]
- He, Y.; Huang, Y.-Y.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Tetracyclines Function as Dual-Action Light-Activated Antibiotics. PLoS ONE 2018, 13, e0196485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krumdieck, S.P.; Boichot, R.; Gorthy, R.; Land, J.G.; Lay, S.; Gardecka, A.J.; Polson, M.I.J.; Wasa, A.; Aitken, J.E.; Heinemann, J.A.; et al. Nanostructured TiO2 Anatase-Rutile-Carbon Solid Coating with Visible Light Antimicrobial Activity. Sci. Rep. 2019, 9, 1883. [Google Scholar] [CrossRef]
- UCL Bacteria Killed by New Light-Activated Coating. Available online: https://www.ucl.ac.uk/news/2020/mar/bacteria-killed-new-light-activated-coating (accessed on 17 June 2021).
- Ikeda, T.; Yamaguchi, H.; Tazuke, S. Molecular Weight Dependence of Antibacterial Activity in Cationic Disinfectants. J. Bioact. Compat. Polym. 1990, 5, 31–41. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.; de Melo Carrasco, L. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jampala, S.N.; Sarmadi, M.; Somers, E.B.; Wong, A.C.L.; Denes, F.S. Plasma-Enhanced Synthesis of Bactericidal Quaternary Ammonium Thin Layers on Stainless Steel and Cellulose Surfaces. Langmuir 2008, 24, 8583–8591. [Google Scholar] [CrossRef]
- Zhong, L.J.; Pang, L.Q.; Che, L.M.; Wu, X.E.; Chen, X.D. Nafion Coated Stainless Steel for Anti-Biofilm Application. Colloids Surf. B Biointerfaces 2013, 111, 252–256. [Google Scholar] [CrossRef]
- Maillard, J.-Y. Antimicrobial biocides in the healthcare environment: Efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Abel zur Wiesch, P.; Busa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Bonhoeffer, S. Antagonism between Bacteriostatic and Bactericidal Antibiotics Is Prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef] [Green Version]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and Antimicrobial Biomaterials: An Overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive Antibacterial Biomaterial Surfaces and Their Applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef]
- Iglic, A.; Garcia-Saez, A.J.; Rappolt, M. Advances in Biomembranes and Lipid Self-Assembly; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-08-102857-5. [Google Scholar]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The Global Threat of Antimicrobial Resistance: Science for Intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [Green Version]
- EARS-Net Surveillance Data. Summary of the Latest Data on Antibiotic Resistance in the European Union. Available online: https://www.ecdc.europa.eu/en/publications-data/summary-latest-data-antibiotic-resistance-european-union (accessed on 18 June 2021).
- Fuglsang, C.C.; Johansen, C.; Christgau, S.; Adler-Nissen, J. Antimicrobial Enzymes: Applications and Future Potential in the Food Industry. Trends Food Sci. Technol. 1995, 6, 390–396. [Google Scholar] [CrossRef]
- Oldham, E.R.; Daley, M.J. Lysostaphin: Use of a Recombinant Bactericidal Enzyme as a Mastitis Therapeutic. J. Dairy Sci. 1991, 74, 4175–4182. [Google Scholar] [CrossRef]
- Faure, E.; Vreuls, C.; Falentin-Daudré, C.; Zocchi, G.; Van de Weerdt, C.; Martial, J.; Jérôme, C.; Duwez, A.-S.; Detrembleur, C. A Green and Bio-Inspired Process to Afford Durable Anti-Biofilm Properties to Stainless Steel. Biofouling 2012, 28, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme Immobilization: An Update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, D.; Olívia Pereira, M. Mini-Review: Antimicrobial Peptides and Enzymes as Promising Candidates to Functionalize Biomaterial Surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial Peptides: Key Components of the Innate Immune System. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial Peptide Melimine Coating for Titanium and Its in Vivo Antibacterial Activity in Rodent Subcutaneous Infection Models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Majhi, S.; Arora, A.; Mishra, A. Antibacterial Activity of Antimicrobial Peptide (AMP) Grafted Polystyrene Surface. In Advances in Polymer Sciences and Technology; Gupta, B., Ghosh, A.K., Suzuki, A., Rattan, S., Eds.; Springer: Singapore, 2018; ISBN 9789811325670. [Google Scholar]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-Leaching Antimicrobial Surfaces through Polydopamine Bio-Inspired Coating of Quaternary Ammonium Salts or an Ultrashort Antimicrobial Lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Maleki, H.; Rai, A.; Pinto, S.; Evangelista, M.; Cardoso, R.M.S.; Paulo, C.; Carvalheiro, T.; Paiva, A.; Imani, M.; Simchi, A.; et al. High Antimicrobial Activity and Low Human Cell Cytotoxicity of Core–Shell Magnetic Nanoparticles Functionalized with an Antimicrobial Peptide. ACS Appl. Mater. Interfaces 2016, 8, 11366–11378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Santos, C.M.; Kumar, A.; Zhao, M.; Lopez, A.I.; Qin, G.; McDermott, A.M.; Cai, C. “Click” Immobilization on Alkylated Silicon Substrates: Model for the Study of Surface Bound Antimicrobial Peptides. Chem. Eur. J. 2011, 17, 2656–2665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- da Silva Sabo, S.; Vitolo, M.; González, J.M.D.; de Souza Oliveira, R.P. Overview of Lactobacillus Plantarum as a Promising Bacteriocin Producer among Lactic Acid Bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef]
- Verma, A.K.; Banerjee, R.; Dwivedi, H.P.; Juneja, V.K. BACTERIOCINS | Potential in Food Preservation. In Encyclopedia of Food Microbiology; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-384733-1. [Google Scholar]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Camargo, A.C.; Todorov, S.D.; Chihib, N.E.; Drider, D.; Nero, L.A. Lactic Acid Bacteria (LAB) and Their Bacteriocins as Alternative Biotechnological Tools to Control Listeria Monocytogenes Biofilms in Food Processing Facilities. Mol. Biotechnol. 2018, 60, 712–726. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible Films and Coatings Containing Bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre Novaes, L.C.; Pessoa, A. Nisin. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; InTech: London, UK, 2015; ISBN 978-953-51-2232-6. [Google Scholar]
- Faure, E.; Lecomte, P.; Lenoir, S.; Vreuls, C.; Van De Weerdt, C.; Archambeau, C.; Martial, J.; Jérôme, C.; Duwez, A.-S.; Detrembleur, C. Sustainable and Bio-Inspired Chemistry for Robust Antibacterial Activity of Stainless Steel. J. Mater. Chem. 2011, 21, 7901. [Google Scholar] [CrossRef]
- Vreuls, C.; Zocchi, G.; Thierry, B.; Garitte, G.; Griesser, S.S.; Archambeau, C.; Van de Weerdt, C.; Martial, J.; Griesser, H. Prevention of Bacterial Biofilms by Covalent Immobilization of Peptides onto Plasma Polymer Functionalized Substrates. J. Mater. Chem. 2010, 20, 8092. [Google Scholar] [CrossRef]
- Vreuls, C.; Zocchi, G.; Garitte, G.; Archambeau, C.; Martial, J.; Van de Weerdt, C. Biomolecules in Multilayer Film for Antimicrobial and Easy-Cleaning Stainless Steel Surface Applications. Biofouling 2010, 26, 645–656. [Google Scholar] [CrossRef]
- Cao, P.; Li, W.-W.; Morris, A.R.; Horrocks, P.D.; Yuan, C.-Q.; Yang, Y. Investigation of the Antibiofilm Capacity of Peptide-Modified Stainless Steel. R. Soc. Open Sci. 2018, 5, 172165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duday, D.; Vreuls, C.; Moreno, M.; Frache, G.; Boscher, N.D.; Zocchi, G.; Archambeau, C.; Van De Weerdt, C.; Martial, J.; Choquet, P. Atmospheric Pressure Plasma Modified Surfaces for Immobilization of Antimicrobial Nisin Peptides. Surf. Coat. Technol. 2013, 218, 152–161. [Google Scholar] [CrossRef]
- Mauchauffé, R.; Moreno-Couranjou, M.; Boscher, N.D.; Van De Weerdt, C.; Duwez, A.-S.; Choquet, P. Robust Bio-Inspired Antibacterial Surfaces Based on the Covalent Binding of Peptides on Functional Atmospheric Plasma Thin Films. J. Mater. Chem. B 2014, 2, 5168. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, J.; Bradley, J.W.; McKay, K.; McBride, F.; Donaghy, D.; Raval, R.; D’Sa, R.A. Linker-Free Covalent Immobilization of Nisin Using Atmospheric Pressure Plasma Induced Grafting. J. Mater. Chem. B 2017, 4, 37–45. [Google Scholar] [CrossRef]
- Friedlander, A.; Nir, S.; Reches, M.; Shemesh, M. Preventing Biofilm Formation by Dairy-Associated Bacteria Using Peptide-Coated Surfaces. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Espejo, H.M.; Díaz-Amaya, S.; Stanciu, L.A.; Bahr, D.F. Nisin Infusion into Surface Cracks in Oxide Coatings to Create an Antibacterial Metallic Surface. Mater. Sci. Eng. C 2019, 105, 110034. [Google Scholar] [CrossRef]
- Cao, P.; Du, C.; He, X.; Zhang, C.; Yuan, C. Modification of a Derived Antimicrobial Peptide on Steel Surface for Marine Bacterial Resistance. Appl. Surf. Sci. 2020, 510, 145512. [Google Scholar] [CrossRef]
- Saini, S.; Sillard, C.; Naceur Belgacem, M.; Bras, J. Nisin Anchored Cellulose Nanofibers for Long Term Antimicrobial Active Food Packaging. RSC Adv. 2016, 6, 12422–12430. [Google Scholar] [CrossRef]
- Arakha, M.; Borah, S.M.; Saleem, M.; Jha, A.N.; Jha, S. Interfacial Assembly at Silver Nanoparticle Enhances the Antibacterial Efficacy of Nisin. Free. Radic. Biol. Med. 2016, 101, 434–445. [Google Scholar] [CrossRef]
- Qi, X.; Poernomo, G.; Wang, K.; Chen, Y.; Chan-Park, M.B.; Xu, R.; Chang, M.W. Covalent Immobilization of Nisin on Multi-Walled Carbon Nanotubes: Superior Antimicrobial and Anti-Biofilm Properties. Nanoscale 2011, 3, 1874. [Google Scholar] [CrossRef]
- Dutz, S.; Wojahn, S.; Gräfe, C.; Weidner, A.; Clement, J. Influence of Sterilization and Preservation Procedures on the Integrity of Serum Protein-Coated Magnetic Nanoparticles. Nanomaterials 2017, 7, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asaduzzaman, S.M.; Sonomoto, K. Lantibiotics: Diverse Activities and Unique Modes of Action. J. Biosci. Bioeng. 2009, 107, 475–487. [Google Scholar] [CrossRef]
- Settanni, L.; Corsetti, A. Application of Bacteriocins in Vegetable Food Biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Piper, C.; Hill, C.; Cotter, P.D.; Ross, R.P. Bioengineering of a Nisin A-Producing Lactococcus Lactis to Create Isogenic Strains Producing the Natural Variants Nisin F, Q and Z: Comparing Natural Nisin Variants. Microb. Biotechnol. 2011, 4, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, S.; Hill, C.; Cotter, P.D. Investigating the Importance of Charged Residues in Lantibiotics. Bioeng. Bugs 2010, 1, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Repka, L.M.; Chekan, J.R.; Nair, S.K.; van der Donk, W.A. Mechanistic Understanding of Lanthipeptide Biosynthetic Enzymes. Chem. Rev. 2017, 117, 5457–5520. [Google Scholar] [CrossRef] [PubMed]
- Chandrapati, S.; O’Sullivan, D.J. Procedure for quantifiable assessment of nutritional parameters influencing Nisin production by Lactococcus lactis subsp. lactis. J. Biotechnol. 1998, 63, 229–233. [Google Scholar] [CrossRef]
- Albanese Donatella; Garofalo Francesca; Pilloton Roberto; Capo Salvatore; Malvano Francesca Development of an Antimicrobial Peptide-Based Biosensor for the Monitoring of Bacterial Contaminations. Chem. Eng. Trans. 2019, 75, 61–66. [CrossRef]
- Kuwano, K.; Tanaka, N.; Shimizu, T.; Nagatoshi, K.; Nou, S.; Sonomoto, K. Dual Antibacterial Mechanisms of Nisin Z against Gram-Positive and Gram-Negative Bacteria. Int. J. Antimicrob. Agents 2005, 26, 396–402. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vandamme, E.J. Nisin, A Lantibiotic Produced by Lactococcus Lactis Subsp. Lactis: Properties, Biosynthesis, Fermentation and Applications. In Bacteriocins of Lactic Acid Bacteria; De Vuyst, L., Vandamme, E.J., Eds.; Springer: Boston, MA, USA, 1994; ISBN 978-1-4613-6146-6. [Google Scholar]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from Lactic Acid Bacteria: Purification, Properties and Use as Biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 512–542. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kizhakkedathu, J.; Straus, S. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasper, H.E.; De Kruijff, B.; Breukink, E. Assembly and Stability of Nisin−Lipid II Pores†. Biochemistry 2004, 43, 11567–11575. [Google Scholar] [CrossRef]
- Bauer, R.; Dicks, L.M.T. Mode of Action of Lipid II-Targeting Lantibiotics. Int. J. Food Microbiol. 2005, 101, 201–216. [Google Scholar] [CrossRef]
- van Heusden, H.E.; de Kruijff, B.; Breukink, E. Lipid II Induces a Transmembrane Orientation of the Pore-Forming Peptide Lantibiotic Nisin. Biochemistry 2002, 41, 12171–12178. [Google Scholar] [CrossRef]
- Karam, L.; Jama, C.; Dhulster, P.; Chihib, N.-E. Study of Surface Interactions between Peptides, Materials and Bacteria for Setting up Antimicrobial Surfaces and Active Food Packaging. J. Mater. Environ. Sci. 2013, 798–821. [Google Scholar]
- Breukink, E.; de Kruijff, B. The Lantibiotic Nisin, a Special Case or Not? Biochim. Biophys. Acta Biomembr. 1999, 1462, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Shin, J.M.; Ateia, I.; Paulus, J.R.; Liu, H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Antimicrobial Nisin Acts against Saliva Derived Multi-Species Biofilms without Cytotoxicity to Human Oral Cells. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the Bacteriocin, Nisin. Antonie van Leeuwenhoek 1996, 69, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological Tools for Bio-Preservation and Shelf-Life Extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- Crandall, A.D.; Montville, T.J. Nisin Resistance in Listeria Monocytogenes ATCC 700302 Is a Complex Phenotype. Appl. Environ. Microbiol. 1998, 64, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chihib, N.-E.; Crepin, T.; Delattre, G.; Tholozan, J.-L. Involvement of Cell Envelope in Nisin Resistance of Pectinatus Frisingensis, a Gram-Negative, Strictly Anaerobic Beer-Spoilage Bacterium Naturally Sensitive to Nisin. FEMS Microbiol. Lett. 1999, 177, 167–175. [Google Scholar] [CrossRef]
- Maurício, E.; Rosado, C.; Duarte, M.; Verissimo, J.; Bom, S.; Vasconcelos, L. Efficiency of Nisin as Preservative in Cosmetics and Topical Products. Cosmetics 2017, 4, 41. [Google Scholar] [CrossRef]
- Fernández, L.; Delgado, S.; Herrero, H.; Maldonado, A.; Rodríguez, J.M. The Bacteriocin Nisin, an Effective Agent for the Treatment of Staphylococcal Mastitis During Lactation. J. Hum. Lact. 2008, 24, 311–316. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hage, M.; Akoum, H.; Chihib, N.-E.; Jama, C. Antimicrobial Peptides-Coated Stainless Steel for Fighting Biofilms Formation for Food and Medical Fields: Review of Literature. Coatings 2021, 11, 1216. https://doi.org/10.3390/coatings11101216
Hage M, Akoum H, Chihib N-E, Jama C. Antimicrobial Peptides-Coated Stainless Steel for Fighting Biofilms Formation for Food and Medical Fields: Review of Literature. Coatings. 2021; 11(10):1216. https://doi.org/10.3390/coatings11101216
Chicago/Turabian StyleHage, Mayssane, Hikmat Akoum, Nour-Eddine Chihib, and Charafeddine Jama. 2021. "Antimicrobial Peptides-Coated Stainless Steel for Fighting Biofilms Formation for Food and Medical Fields: Review of Literature" Coatings 11, no. 10: 1216. https://doi.org/10.3390/coatings11101216
APA StyleHage, M., Akoum, H., Chihib, N. -E., & Jama, C. (2021). Antimicrobial Peptides-Coated Stainless Steel for Fighting Biofilms Formation for Food and Medical Fields: Review of Literature. Coatings, 11(10), 1216. https://doi.org/10.3390/coatings11101216