Electroless Ni–P Plating on Mullite Powders and Study of the Mechanical Properties of Its Plasma-Sprayed Coating
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Preparation of the Ni–P-Coated Mullite Powders
2.2.1. Pretreatment of the Mullite Powders
2.2.2. Orthogonal Experimental Design of Electroless Plating
2.2.3. Electroless Plating with Ni–P
2.3. Preparation of Coatings
2.4. Thermal Cycle Test of the Coatings
2.5. Characterization of the Coated Mullite Powders and As-Sprayed Coatings
3. Results and Discussion
3.1. Microstructures and Phase Compositions of the Ni–P/Mullite Composite Powders
3.2. Microstructure and Phase Compositions of the Coatings
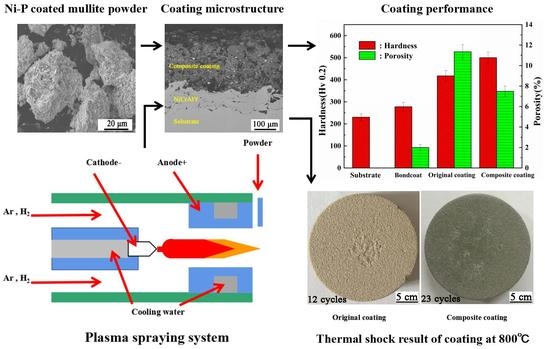
3.3. Hardness and Thermal Conductivity of the Coatings
3.4. Thermal Cycling Resistances of the Coatings
4. Conclusions
- The Ni–P layer could be coated on the surface of mullite powders by electroless plating. The layer of coated powder was uniform and dense, without leakage or aggregation.
- In the electroless plating, Ni2+ was absorbed on the surface of the pretreated mullite and reduced to nucleate growth near the Pd atom, forming an island-like structure. With the reaction, Ni particles grew and aggregated in three dimensions, forming a Ni–P layer film on the surface of the mullite. The Ni particles formed on the surface of the film served as activation centers for reaction continuation.
- Compared to the original mullite coating, the composite coating with the Ni–P layer exhibited a better wettability, lower porosity, higher hardness, and larger binding force under the condition that the thermal conductivity is not significantly different from that of the original mullite coating. This study provides a feasible approach for the applications of mullite as a wear-resistance material of harsh environments, environmental barrier coatings (EBCs) of the ceramic matrix composite material and TBCs of lower-temperature environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Vaßen, R.; Kagawa, Y.; Subramanian, R.; Zombo, P.; Zhu, D. Testing and evaluation of thermal-barrier coatings. MRS Bull. 2012, 37, 911–916. [Google Scholar] [CrossRef]
- Ghasemi, R.; Shoja-Razavi, R.; Mozafarinia, R.; Jamali, H. The influence of laser treatment on thermal shock resistance of plasma-sprayed nanostructured yttria stabilized zirconia thermal barrier coatings. Ceram. Int. 2014, 40, 347–355. [Google Scholar] [CrossRef]
- Narimani, N.; Saremi, M. A study on the oxidation resistance of electrodeposited and nanostructured YSZ thermal barrier ceramic coatings. Ceram. Int. 2015, 41, 13810–13816. [Google Scholar] [CrossRef]
- Jam, A.; Derakhshandeh, S.M.R.; Rajaei, H.; Pakseresht, A.H. Evaluation of microstructure and electrochemical behavior of dual-layer NiCrAlY/mullite plasma sprayed coating on high silicon cast iron alloy. Ceram. Int. 2017, 43, 14146–14155. [Google Scholar] [CrossRef]
- Hu, X.X.; Xu, F.F.; Li, K.W.; Jin, G.; Xu, Y.; Zhao, X.Q. Water vapor corrosion behavior and failure mechanism of plasma sprayed mullite/Lu2Si2O7-Lu2SiO5 coatings. Ceram. Int. 2018, 44, 14177–14185. [Google Scholar] [CrossRef]
- Saravanan, S.; Kumar, C.R.; Pugazhendhi, A.; Brindhadevic, K. Role of thermal barrier coating and porous medium combustor for a diesel engine: An experimental study. Fuel 2020, 280, 118597. [Google Scholar] [CrossRef]
- di Girolamo, G.; Blasi, C.; Pilloni, L.; Schioppa, M. Microstructural and thermal properties of plasma sprayed mullite coatings. Ceram. Int. 2010, 36, 1389–1395. [Google Scholar] [CrossRef]
- Li, S.J.; Zhao, X.Q.; Hou, G.L.; Deng, W.; An, Y.L.; Zhou, H.D.; Chen, J.M. Thermomechanical properties and thermal cycle resistance of plasma-sprayed mullite coating and mullite/zirconia composite coatings. Ceram. Int. 2016, 42, 17447–17455. [Google Scholar] [CrossRef]
- Rangaraj, S.; Kokini, K. Interface thermal fracture in functionally graded zirconia–mullite–bond coat alloy thermal barrier coatings. Acta Mater. 2003, 51, 251–267. [Google Scholar] [CrossRef]
- Bolelli, G.; Cannillo, V.; Lugli, C.; Lusvarghi, L.; Manfredini, T. Plasma-sprayed graded ceramic coatings on refractory materials for improved chemical resistance. J. Eur. Ceram. Soc. 2006, 26, 2561–2579. [Google Scholar] [CrossRef]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.F.; Jin, G.; Gao, Z.H.; Jin, J.N.; Cai, Z.B.; Fang, Y.C. Ceramic Fibers Reinforced Functionally Graded Thermal Barrier Coatings. Adv. Eng. Mater. 2017, 19, 1700149. [Google Scholar] [CrossRef]
- Zhang, X.F.; Song, J.B.; Deng, Z.Q.; Wang, C.; Niu, S.P.; Liu, G.; Deng, C.M.; Deng, C.G.; Liu, M.; Zhou, K.S.; et al. Interface evolution of Si/Mullite/Yb2SiO5 PS-PVD environmental barrier coatings under high temperature. J. Eur. Ceram. Soc. 2020, 40, 1478–1487. [Google Scholar] [CrossRef]
- Vakilifard, H.; Ghasemi, R.; Rahimipour, M. Hot corrosion behaviour of plasma-sprayed functionally graded thermal barrier coatings in the presence of Na2SO4 + V2O5 molten salt. Surf. Coat. Technol. 2017, 326, 238–246. [Google Scholar] [CrossRef]
- Luo, H.; Leitch, M.; Zeng, H.B.; Luo, J.L. Characterization of microstructure and properties of electroless duplex Ni-W-P/Ni-P nano-ZrO2 composite coating. Mater. Today. Phys. 2018, 4, 36–42. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Q.; Yang, P.; Chen, H.H.; Hong, H.P.; Zhang, W.T.; Ren, J. Preparation of nickel coating on ZTA particles by electroless plating. Ceram. Int. 2018, 44, 11013–11021. [Google Scholar] [CrossRef]
- Liu, W.N.; Qiao, X.J.; Liu, S.; Shi, S.M.; Liang, K.; Tang, L. A new process for pre-treatment of electroless copper plating on the surface of mica powders with ultrasonic and nano-nickel. J. Alloy. Compd. 2019, 791, 613–620. [Google Scholar] [CrossRef]
- Kong, D.H.; Zuo, X.Q.; Wang, Y.W.; Zhou, Y. Microstructure and thermal decomposition property of Ni-P/Cr2N composite powder by electroless plating. Adv. Powder Technol. 2018, 29, 1433–1438. [Google Scholar] [CrossRef]
- Dai, H.; Li, H.; Wang, F. Electroless Ni–P coating preparation of conductive mica powder by a modified activation process. Appl. Surf. Sci. 2006, 253, 2474–2480. [Google Scholar] [CrossRef]
- Yuan, X.T.; Sun, D.B.; Yu, H.Y.; Meng, H.M.; Fan, Z.S.; Wang, X.D. Preparation of amorphous-nanocrystalline composite structured Ni–P electrodeposits. Surf. Coat. Technol. 2007, 202, 294–300. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.L.; Matsuda, K.; Zou, Y. Microstructure evolution and corrosion resistance of Ni–Cu–P amorphous coating during crystallization process. Appl. Surf. Sci. 2019, 484, 835–844. [Google Scholar] [CrossRef]
- Ghavidel, N.; Allahkaram, S.R.; Naderi, R.; Barzegar, M.; Bakhshandeh, H. Corrosion and wear behavior of an electroless Ni-P/nano-SiC coating on AZ31 Mg alloy obtained through environmentally-friendly conversion coating. Surf. Coat. Technol. 2020, 382, 125156. [Google Scholar] [CrossRef]
- van den Brand, J.; Sloof, W.G.; Terryn, H.; de Wit, J.H.W. Correlation between hydroxyl fraction and O/Al atomic ratio as determined from XPS spectra of aluminium oxide layers. Surf. Interface. Anal. 2004, 36, 81–88. [Google Scholar] [CrossRef]
- An, Y.L.; Chen, J.M.; Zhou, H.D.; Liu, G. Microstructure and thermal cycle resistance of plasma sprayed mullite coatings made from secondary mullitized natural andalusite powder. Surf. Coat. Technol. 2010, 205, 1897–1903. [Google Scholar] [CrossRef]
- Li, S.J.; Xi, X.; Hou, G.L.; An, Y.L.; Zhao, X.Q.; Zhou, H.D.; Chen, J.M. Preparation of plasma sprayed mullite coating on stainless steel substrate and investigation of its environmental dependence of friction and wear behavior. Tribol. Int. 2015, 91, 32–39. [Google Scholar] [CrossRef]
- Rad, M.R.; Farrahi, G.H.; Azadi, M.; Ghodrati, M. Stress analysis of thermal barrier coating system subjected to out-of-phase thermo-mechanical loadings considering roughness and porosity effect. Surf. Coat. Technol. 2015, 262, 77–86. [Google Scholar]
- See, A.; Hassan, J.; Hashim, M.; Wahab, Z.A. Thermal diffusivity of kaolinite–mullite ceramic matrix composite with silicon nitride nanoparticle filler. Thermochim. Acta. 2014, 593, 76–81. [Google Scholar] [CrossRef]
- Izadinia, M.; Soltani, R.; Sohi, M.H. Effect of segmented cracks on TGO growth and life of thick thermal barrier coating under isothermal oxidation conditions. Ceram. Int. 2020, 46, 7475–7481. [Google Scholar] [CrossRef]
- Foroushani, M.H.; Shamanian, M.; Salehi, M.; Davar, F. Porosity analysis and oxidation behavior of plasma sprayed YSZ and YSZ/LaPO4 abradable thermal barrier coatings. Ceram. Int. 2016, 42, 15868–15875. [Google Scholar] [CrossRef]
- Liu, P.F.; Jiang, P.; Sun, Y.L.; Xu, R.; Wang, T.J.; Zhang, W.X. Numerical Analysis of stress evolution in thermal barrier coating system during two-stage growth of heterogeneous oxide. Ceram. Int. 2021, 47, 14311–14319. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, G.X.; Mu, R.D.; He, L.M.; Xu, Z.H.; Dai, J.W. Effects of Er stabilization on thermal property and failure behavior of Gd2Zr2O7 thermal barrier coatings. Corros. Sci. 2021, 185, 109418. [Google Scholar] [CrossRef]
- Hussain, N.; Qayyum, F.; Pasha, R.A.; Shah, M. Development of multi-physics numerical simulation model to investigate thermo-mechanical fatigue crack propagation in an autofrettaged gun barrel. Def. Technol. 2021, 17, 1579–1591. [Google Scholar] [CrossRef]
- Mukhtar, F.; Qayyum, F.; Elahi, H.; Shah, M. Studying the effect of thermal fatigue on multiple cracks propagating in an SS316L thin flange on a shaft specimen using a multi-physics numerical simulation model. J. Mech. Eng. 2019, 65, 565–573. [Google Scholar] [CrossRef]












| Element | C | Si | Mn | P | S | Ni | Cr | N | Fe |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.07 | 0.37 | 1.27 | 0.03 | 0.01 | 8.02 | 17.2 | 0.06 | Balance |
| Level | Factor | |||
|---|---|---|---|---|
| Nickel Sulfate Hexahydrate (A)/(g/L) | Sodium Hypophosphite (B)/(g/L) | Temperature (C)/℃ | pH (D) | |
| 1 | 15 | 10 | 40 | 4.5 |
| 2 | 20 | 20 | 60 | 5 |
| 3 | 25 | 30 | 80 | 5.5 |
| The optimal factor | 20 | 30 | 80 | 5.5 |
| Parameters | NiCrAlY Coating | Mullite Coating |
|---|---|---|
| Arc current (A) | 600 | 500 |
| Voltage (V) | 60 | 70 |
| Argon gas flow rate (L/min) | 40 | 45 |
| Hydrogen gas flow rate (L/min) | 15 | 15 |
| Powder gas flow rate (L/min) | 5 | 8 |
| Spray distance (mm) | 90 | 90 |
| Spray angle (°) | 90 | 90 |
| Gun speed (m/s) | 0.3 | 0.2 |
| Sample | Density (g/cm3) | Specific Heat Capacity (kJ/kg·K) | Thermal Diffusivity (mm2/S) | Thermal Conductivity (W/m·K) |
|---|---|---|---|---|
| OC | 2.744 | 1.353 | 0.904 | 3.356 |
| NC | 3.015 | 0.976 | 1.203 | 3.540 |
| Oc/Nc (%) | 91.01 | 138.63 | 75.15 | 94.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Zhang, P.; Sun, P.; Niu, X.; Hu, C. Electroless Ni–P Plating on Mullite Powders and Study of the Mechanical Properties of Its Plasma-Sprayed Coating. Coatings 2022, 12, 18. https://doi.org/10.3390/coatings12010018
Chen K, Zhang P, Sun P, Niu X, Hu C. Electroless Ni–P Plating on Mullite Powders and Study of the Mechanical Properties of Its Plasma-Sprayed Coating. Coatings. 2022; 12(1):18. https://doi.org/10.3390/coatings12010018
Chicago/Turabian StyleChen, Kaiwang, Penglin Zhang, Pengfei Sun, Xianming Niu, and Chunlian Hu. 2022. "Electroless Ni–P Plating on Mullite Powders and Study of the Mechanical Properties of Its Plasma-Sprayed Coating" Coatings 12, no. 1: 18. https://doi.org/10.3390/coatings12010018
APA StyleChen, K., Zhang, P., Sun, P., Niu, X., & Hu, C. (2022). Electroless Ni–P Plating on Mullite Powders and Study of the Mechanical Properties of Its Plasma-Sprayed Coating. Coatings, 12(1), 18. https://doi.org/10.3390/coatings12010018