Corrosion of Fe-Cr-Si Alloys in Oxidizing and Sulphidizing-Oxidizing Atmospheres
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
3.1. Prepared Sample
3.2. Corrosion Kinetics
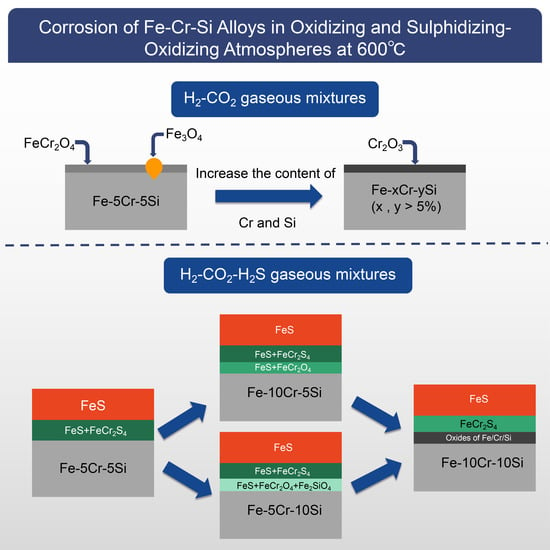
3.3. Scale Morphology
3.3.1. Microstructures of the Oxide Scales on Fe-Cr-Si Alloys Oxidized in the Oxidizing Atmosphere
3.3.2. Microstructures of the Scales on Fe-Cr-Si Alloys Corroded in the Oxidizing-Sulphidizing Atmosphere
4. Discussion
4.1. Oxidation Mechanism in the H2-CO2 Mixture
4.2. Corrosion Mechanism in the H2-CO2-H2S Mixture
4.3. The Effect of Sulphur
4.4. The Effect of Chromium
5. Conclusions
- Cr promoted the formation of a chromium-rich oxide layer on the Fe-Si alloys in a H2-CO2 atmosphere, thus significantly improving the corrosion resistance of the alloys, especially when the content was more than 5 at.%.
- Introducing H2S gas into the H2-CO2 atmosphere made the Fe-Cr-Si alloys form loose and easy-to-peel FeS outer layers and FeCr2S4 inner layers instead of a dense oxide layer, thus significantly intensifying the corrosion of the alloy.
- Attributable to the failure of Cr in promoting the selective oxidation of Si, the introduction of Cr to the Fe-Si alloys had little beneficial effect in improving the corrosion resistance of iron-based alloys in a H2-CO2-H2S atmosphere.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, H.L.; Datta, P.K.; Griffin, D.; Aljarany, A.; Burnell-Gray, J.S. Oxidation and Sulfidation Behavior of AlTiN-Coated Ti–46.7Al–1.9W–0.5Si Intermetallic with CrN and NbN Diffusion Barriers at 850 °C. Oxid. Met. 2003, 60, 29–46. [Google Scholar] [CrossRef]
- Yong, M. Cause Analysis and Solution Method of Welding Seam Leakage of Blocking Plate Valve for Boiler Main Stream Pipe. Hunan Electr. Pow. 2020, 40, 32–35. [Google Scholar]
- Xie, A.; Chen, H. Discussion on safety management of important bolts in hydropower plants. Hunan Electr. Pow. 2013, 33, 54–62. [Google Scholar]
- Li, W.; Xie, Y.; Li, D.; Hu, J.; Chen, H. Dew point corrosion of air preheater and preventive measures. Hunan Electr. Pow. 2016, 1, 3–9. [Google Scholar]
- Li, W.; Xie, Y.; Liu, Y.; Wu, T.; Long, Y. Study on corrosion resistance of NiTi memory alloy butterfly gasket. Hunan Electr. Pow. 2017, 37, 29–32. [Google Scholar]
- Wan, D.; Qi, F.; Zhou, H.; Zhao, M.; Duan, X.; Huang, Y. Analysis of a typical fault of 10 kV power cable cluster burning. Hunan Electr. Pow. 2020, 40, 84–89. [Google Scholar]
- Young, D. High Temperature Oxidation and Corrosion of Metals; Elsevier: Oxford, UK, 2008. [Google Scholar]
- Richardson, T. Shreir’s Corrosion; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Huczkowski, P.; Olszewski, T.; Schiek, M.; Lutz, B.; Holcomb, G.R.; Shemet, V.; Nowak, W.; Meier, G.H.; Singheiser, L.; Quadakkers, W.Q. Effect of SO2 on oxidation of metallic materials in CO2/H2O-rich gases relevant to oxyfuel environments. Mater. Corros. 2013, 65, 121–131. [Google Scholar] [CrossRef]
- Niu, Y.; Gesmundo, F.; Viani, F. The oxidation-sulfidation of Fe-Nb alloys at 600-800oC in H2-H2S-CO2 mixtures. Corros. Sci. 1994, 36, 1885–1906. [Google Scholar] [CrossRef]
- Niu, Y.; Gesmundo, F.; Yan, R.; Wu, W. The corrosion of Fe-15 wt% Y and Fe-30 wt% Y in sulfidizing-oxidizing atmospheres at 600–800 °C. Corros. Sci. 1999, 41, 989–1012. [Google Scholar] [CrossRef]
- Fu, G.; Niu, Y.; Wu, W. High temperature oxidation of powder metallurgy two phase Cu-Cr alloys under low oxygen pressure. T. Nonferr. Metal. Soc. 2000, 10, 353–357. [Google Scholar]
- Xie, A.; Long, Y. Finite element analysis of stainless steel runner blade in francis turbine. Hunan Electr. Pow. 2011, 31, 14–16. [Google Scholar]
- Liu, L.; Guo, Q.; Zeng, C.; Niu, Y. Corrosion of binary Fe-Si alloys in reducing oxidizing and sulfidizing-oxidizing atmospheres at 700 °C. Oxid. Met. 2014, 81, 477–502. [Google Scholar] [CrossRef]
- Stott, F.; Gabriel, G.; Wood, G. The influence of silicon on the high-temperature oxidation of nickel. Oxid. Met. 1987, 28, 329–345. [Google Scholar] [CrossRef]
- Tomlinson, W.; Yates, J. High temperature oxidation kinetics of Cu-Si alloys containing up to 4.75wt.% Si in p(O2)=0.01atm and pure CO2. Oxid. Met. 1978, 12, 323–329. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.; Liu, S.; Ni, C.; Niu, Y. Anomalous oxidation of Fe-Si alloys under a low oxygen pressure at 800 °C. Corros. Sci. 2015, 98, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, W.; Zhu, R. Simultaneous sulphidation and oxidation of Fe-25 wt.%Cr and Silicon-containing Fe-25 wt.%Cr alloys in H2-H2O-H2S gas mixtures at high temperatures. Mat. Sci. Eng. C 1990, 125, 223–233. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Y. Corrosion of Fe-Si alloys in reducing oxidizing and sulfidizing-oxidizing atmospheres at 600 °C. J. Soc. Corros. Protec. 2014, 34, 307–314. [Google Scholar]
- Bamba, G.; Wouters, Y.; Galerie, A.; Charlot, F.; Dellali, A. Thermal oxidation kinetics and oxide scale adhesion of 15Cr alloys in function of their silicon content. Acta Mater. 2006, 54, 3917–3922. [Google Scholar] [CrossRef]
- Guo, G.; Liu, S.; Wu, X.; Liu, L.; Niu, Y. Scaling behavior of two Fe-xCr-5Si alloys under high and low oxygen pressures at 700 °C. Corros. Sci. 2015, 100, 579–588. [Google Scholar] [CrossRef]
- Robertson, J.; Manning, M.I. Healing layer formation in Fe-Cr-Si ferritic steels. Mater. Sci. Technol. 1989, 5, 741–753. [Google Scholar] [CrossRef]
- Wagner, C. Passivity and inhibition during the oxidation of metals at elevated temperatures. Corros. Sci. 1965, 5, 751. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Gesmundo, F.; Niu, Y. The Effect of Cr on the Oxidation of Ni–10at% Al in 1atm O at 900–1000 °C. Oxid. Met. 2006, 65, 151–165. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, Y. Effect of Chromium on the Oxidation of a Fe-10 Al Alloy at 1000 °C. Adv. Mater. Process. 2004, 4, 685–688. [Google Scholar]
- Zhang, X.; Gao, C.; Niu, Y. Effect of 5%Cr on oxidation of Ni-10Al in 0.1MPa O2 at 900 °C and 1000 °C. Trans. Nonferrous Met. Soc. China 2006, 3, 2042–2045. [Google Scholar]
- Jiao, S.; Wen, S.; Ren, T.; Li, L.; Liu, R.; Lin, L. Impact of third element effect on the surface internal and external oxidations of TWIP steel during annealing. Shanghai Met. 2018, 02, 40. [Google Scholar]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagrams; ASM International: Geauga, OH, USA, 1995. [Google Scholar]
- Zhang, Y.; Ma, Z.; Wang, W.; Li, K.; Li, Y.T.; Yang, W. Molecular dynamics simulations of interaction and very first step oxidation in the surface of ferritic Fe-Cr alloy. Comp. Mater. Sci. 2021, 195, 110500. [Google Scholar] [CrossRef]
- Hao, M.; Sun, B.; Wang, H. High-temperature oxidation behavior of Fe–1Cr–0.2 Si Steel. Materials 2020, 13, 509. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Fu, G.; Gesmundo, F. The corrosion of a Fe-15 wt.% Ce alloy in coal gasification type atmospheres at 600 to 800 °C. J. Phase Equilibria Differ. 2002, 23, 61–67. [Google Scholar] [CrossRef]
- Zhou, W. Treatment and Analysis of Water Leakage Fault on Coil Winding for Double Water Internal Cooling Generator Rotor. Hunan Electr. Pow. 2020, 40, 36–38. [Google Scholar]
- Gude, A.; Mehrer, H. Defect Diffus. Forum 1997, 143, 351. [Google Scholar]
- Mrowec, S.; Przybylski, K. Transport properties of sulfide scales and sulfidation of metals and alloys. Oxid. Met. 1985, 23, 107–139. [Google Scholar] [CrossRef]
- Mrowec, S. Mechanism of high-temperature sulphide corrosion of metals and alloys. Mater. Corros. 1980, 31, 371–386. [Google Scholar] [CrossRef]
- Meier, G.H.; Jung, K.; Mu, N.; Yanar, N.M.; Pettit, F.S.; Abellán, J.P.; Olszewski, T.; Hierro, L.N.; Quadakkers, W.J.; Holcomb, G. Effect of alloy composition and exposure conditions on the selective oxidation behavior of ferritic Fe-Cr and Fe-Cr-X alloys. Oxid. Met. 2010, 74, 319–340. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.; Lee, D. Corrosion of Fe-2.25%Cr-1%Mo steels at 600-800oC in N2/H2O/H2S atmospheres. Energy Procedia 2012, 14, 1837–1842. [Google Scholar] [CrossRef]










| 600 | H2 | CO2 | H2S | P (O2) | P (S2) |
|---|---|---|---|---|---|
| O | 13.9 | 86.1 | - | 10−24 | - |
| OS | 44.13 | 54.35 | 1.52 | 10−24 | 10−8 |
| Nominal | Actual |
|---|---|
| Fe-4.77/5Cr-2.57/5Si | Fe-4.69/4.9Cr-2.45/4.77Si |
| Fe-9.59/10Cr-2.58/5Si | Fe-9.5/9.93Cr-2.53/4.91Si |
| Fe-4.91/5Cr-5.28/10Si | Fe-4.85/4.95Cr-5.2/9.85Si |
| Fe-9.85/10Cr-5.3/10Si | Fe-9.71/9.87Cr-5.23/9.82Si |
| Elements | O | Si | S | Cr | Fe | |
|---|---|---|---|---|---|---|
| Points | ||||||
| 1 | 0 | 11 | 0 | 10 | 79 | |
| 2 | 0 | 24 | 0 | 3 | 73 | |
| 3 | 46 | 18.8 | 16 | 8 | 11 | |
| 4 | 0 | 0 | 59 | 27 | 14 | |
| 5 | 0 | 0 | 55 | 1 | 44 | |
| 6 | 0 | 0 | 55 | 0 | 45 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Xu, C.; Chen, K.; Liu, L.; Yang, H.; Cheng, Q.; Zeng, M. Corrosion of Fe-Cr-Si Alloys in Oxidizing and Sulphidizing-Oxidizing Atmospheres. Coatings 2022, 12, 1588. https://doi.org/10.3390/coatings12101588
Li W, Xu C, Chen K, Liu L, Yang H, Cheng Q, Zeng M. Corrosion of Fe-Cr-Si Alloys in Oxidizing and Sulphidizing-Oxidizing Atmospheres. Coatings. 2022; 12(10):1588. https://doi.org/10.3390/coatings12101588
Chicago/Turabian StyleLi, Wenbo, Chenghao Xu, Ken Chen, Lanlan Liu, Haiyun Yang, Qiao Cheng, and Minyu Zeng. 2022. "Corrosion of Fe-Cr-Si Alloys in Oxidizing and Sulphidizing-Oxidizing Atmospheres" Coatings 12, no. 10: 1588. https://doi.org/10.3390/coatings12101588
APA StyleLi, W., Xu, C., Chen, K., Liu, L., Yang, H., Cheng, Q., & Zeng, M. (2022). Corrosion of Fe-Cr-Si Alloys in Oxidizing and Sulphidizing-Oxidizing Atmospheres. Coatings, 12(10), 1588. https://doi.org/10.3390/coatings12101588