Natural Dyes Used as Organic Coatings UV Protecting for Food Packages
Abstract
:1. Introduction
2. Materials and Methods
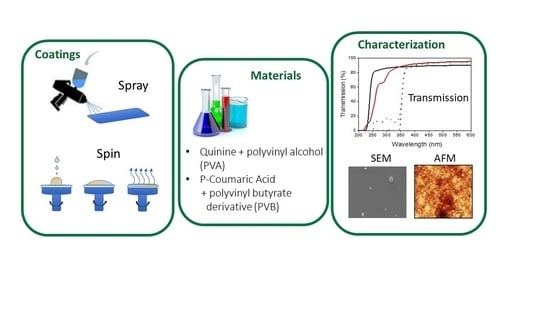
2.1. Materials
2.2. Coatings Preparation
2.3. Samples Characterization
3. Results and Discussion
Spectroscopical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brody, A.L.; Bugusu, B.; Han, J.H.; Sand, C.K.; McHugh, T.H. Innovative food packaging solutions. J. Food Sci. 2008, 73, R107–R116. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, M.; Vassallo, E.; Aloisio, M.; Brasca, M.; Chen, H.; Firpo, G.; Ghezzi, F.; Morandi, S.; Pietralunga, S.M.; Silvetti, T.; et al. Plasma sputtered tungsten oxide thin film on poly(lactic acid) for food packaging applications. Coatings 2021, 11, 1281. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Liu, H.; Shang, S.; Song, J.; Wang, D. Extraction and comparison of carboxylated cellulose nanocrystals from bleached sugarcane bagasse pulp using two different oxidation methods. Carbohydr Polym. 2016, 138, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Alzate Marin, J.C.; Rivero, S.; Pinotti, A.; Caravelli, A.; Zaritzky, N.E. Microstructural behaviors of matrices based on polylactic acid and polyhydroxyalkanoates. J. Agric. Food Chem. 2018, 66, 10033–10040. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Kazemi, Y.; Ding, W.; Ameli, A.; Park, C.B. Poly(Lactic Acid)-Based in situ microfibrillar composites with enhanced crystallization kinetics, mechanical properties, rheological behavior, and foaming ability. Biomacromolecules 2015, 16, 3925–3935. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Bollani, M.; Maccagnan, A.; Piergiovanni, L. Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 2013, 5, 2491–2504. [Google Scholar] [CrossRef] [Green Version]
- Cyras, V.; Commisso, M.; Vázquez, A. Biocomposites based on renewable resource: Acetylated and non acetylated cellulose cardboard coated with polyhydroxybutyrate. Polymer 2009, 50, 6274–6280. [Google Scholar] [CrossRef]
- Mensitieri, G.; Di, M.E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Lannace, S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]
- Socaciu, M.-I.; Fogarasi, M.; Simon, E.L.; Semeniuc, C.A.; Socaci, S.A.; Podar, A.S.; Vodnar, D.C. Effects of whey protein isolate-based film incorporated with tarragon essential oil on the quality and shelf-life of refrigerated brook trout. Foods 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Peelman, N.; Ragaert, P.; Meulenaer, B.D.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devliegherea, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Narayanan, M.; Loganathan, S.; Valapa, R.B.; Thomas, S.; Varghese, T.O. UV protective poly(lactic acid)/rosin films for sustainable packaging. Int. J. Biol. Macromol. 2017, 99, 37–45. [Google Scholar] [CrossRef]
- Oguzlu, H.; Jiang, F. Nanopolysaccharides in surface coating. In Advanced Functional Materials from Nanopolysaccharides; Lin, N., Tang, J.T., Dufresne, A., Tam, M.K.C., Eds.; Springer: Singapore, 2019; pp. 283–319. [Google Scholar] [CrossRef]
- Yezhi, F.; Dudley, E.G. Antimicrobial-coated films as food packaging: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3404–3437. [Google Scholar]
- Sunita, J.; Pant, D.D. Ground and excited state dipole moments of quinine sulfate dication: Solvatochromic shift of absorption and fluorescence spectra. J. Mol. Liq. 2012, 172, 125–129. [Google Scholar] [CrossRef]
- Putschögl, M.; Zirak, P.; Penzkofer, A. Absorption and emission behaviour of trans-p-coumaric acid in aqueous solutions and some organic solvents. Chem. Phys. 2008, 343, 107–120. [Google Scholar] [CrossRef]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar J. 2011, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Abazari, M.F.; Nasiri, N.; Karizi, S.Z.; Nejati, F.; Haghi-Aminjan, H.; Norouzi, S.; Piri, P.; Estakhr, L.; Faradonbeh, D.R.; Kohandani, M.; et al. An updated review of various medicinal applications of p-coumaric acid: From antioxidative and anti-inflammatory properties to effects on cell cycle and proliferation. Mini Rev. Med. Chem. 2021, 21, 2187–2201. [Google Scholar] [CrossRef]
- Bollani, M.; Salvalaglio, M.; Benali, A.; Bouabdellaoui, M.; Naffouti, M.; Lodari, M.; Di Corato, S.; Fedorov, A.; Voigt, A.; Fraj, I.; et al. Templated dewetting of single-crystal sub-millimeter-long nanowires and on-chip silicon circuits. Nat. Commun. 2019, 10, 5632. [Google Scholar] [CrossRef] [Green Version]
- Botta, C.; Betti, P.; Pasini, M. Organic nanostructured host–guest materials for luminescent solar concentrators. J. Mater. Chem. A 2013, 1, 510–514. [Google Scholar] [CrossRef]
- Cappelli, A.; Villafiorita-Monteleone, F.; Grisci, G.; Paolino, M.; Razzano, V.; Fabio, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; et al. Highly emissive supramolecular assemblies based on π-stacked polybenzofulvene hosts and a benzothiadiazole guest. J. Mater. Chem. C 2014, 2, 7897–7905. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Kaniuk, Ł.; Metwally, S.; Szewczyk, P.K.; Sroczyk, E.A.; Peer, P.; Lisiecka-Graca, P.; Bailey, R.J.; Bilotti, E.; Stachewicz, U. Nano- and microfiber pvb patches as natural oil carriers for atopic skin treatment. ACS Appl. Bio Mater. 2020, 3, 7666–7676. [Google Scholar] [CrossRef]
- Chui, C.Y.; Mouthuy, P.A.; Ye, H. Direct electrospinning of poly(vinyl butyral) onto human dermal fibroblasts using a portable device. Biotechnol. Lett. 2018, 40, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Parot, J.; Hackley, V.A.; Zou, A.; Johnston, L.J. AFM characterization of cellulose nanocrystal height and width using internal calibration standards. Cellulose 2021, 28, 1933–1946. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virgili, T.; Pasini, M.; Guizzardi, M.; Tizro, N.; Bollani, M. Natural Dyes Used as Organic Coatings UV Protecting for Food Packages. Coatings 2022, 12, 417. https://doi.org/10.3390/coatings12030417
Virgili T, Pasini M, Guizzardi M, Tizro N, Bollani M. Natural Dyes Used as Organic Coatings UV Protecting for Food Packages. Coatings. 2022; 12(3):417. https://doi.org/10.3390/coatings12030417
Chicago/Turabian StyleVirgili, Tersilla, Mariacecilia Pasini, Michele Guizzardi, Negar Tizro, and Monica Bollani. 2022. "Natural Dyes Used as Organic Coatings UV Protecting for Food Packages" Coatings 12, no. 3: 417. https://doi.org/10.3390/coatings12030417
APA StyleVirgili, T., Pasini, M., Guizzardi, M., Tizro, N., & Bollani, M. (2022). Natural Dyes Used as Organic Coatings UV Protecting for Food Packages. Coatings, 12(3), 417. https://doi.org/10.3390/coatings12030417