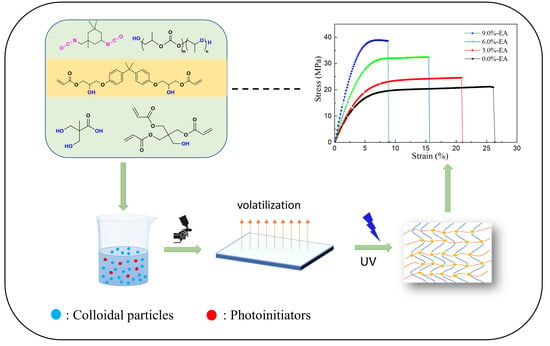
Preparation and Properties of Novel Modified Waterborne Polyurethane Acrylate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydroxyl Value of Epoxy Acrylate
2.3. Preparation of Modified Polyurethane Acrylate Emulsion
2.4. Preparation of Film and Coating
2.5. Characterization
3. Results and Discussion
3.1. Hydroxyl Value of Epoxy Acrylate
3.2. Structure Analysis of WPU-EA
3.3. Particle Size and Distribution of WPU-EA Emulsion
3.4. Photopolymerization Kinetics of WPU-EA Resin
3.5. Tensile Properties
3.6. Dynamic Mechanical Analysis (DMA)
3.7. Gel Fraction
3.8. Thermal Stability of the WPU-EA Film
3.9. Performance of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent Advances in Vegetable Oils Based Environment Friendly Coatings: A Review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Mucci, V.L.; Santamaria-Echart, A.; Corcuera, M.Á.; Eceiza, A.; Aranguren, M.I. Waterborne Polyurethane Nanocomposites Based on Vegetable Oil and Microfibrillated Cellulose. J. Appl. Polym. Sci. 2016, 133, 1–12. [Google Scholar] [CrossRef]
- Dong, X.; Ren, J.; Duan, Y.; Wu, D.; Lin, L.; Shi, J.; Jia, R.; Xu, X.; He, X. Preparation and Properties of Green UV-Curable Itaconic Acid Cross-Linked Modified Waterborne Polyurethane Coating. J. Appl. Polym. Sci. 2022, 139, 52042. [Google Scholar] [CrossRef]
- Wu, G.; Zang, H.; Zhang, H. Preparation and Performance of UV-Curable Waterborne Polyurethane Prepared Using Dipentaerythritol Hexaacrylate/Dipropylene Glycol Diacrylate Monomers. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 927–934. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, A.; Liu, S.; Zhao, J.; Jiang, J.; Liu, X. Effect of Silica Nanoparticles on Structure and Properties of Waterborne UV-Curable Polyurethane Nanocomposites. Polym. Bull. 2012, 68, 1469–1482. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, F.; Wang, Y.; Wu, W.; Yang, D.; Guo, Q. UV-Curable Waterborne Polyurethane-Acrylate: Preparation, Characterization and Properties. Prog. Org. Coatings 2012, 73, 47–53. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Saif, M.J. Recent Trends in Environmentally Friendly Water-Borne Polyurethane Coatings: A Review. Korean J. Chem. Eng. 2016, 33, 388–400. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Su, Q.; He, J.; Li, Y.; Xie, J.; Yi, G. Synthesis of a Novel Hyperbranched Polyester with Carboxyl End Groups Applied to UV-Curable Waterborne Coating. J. Coatings Technol. Res. 2021, 18, 259–269. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Zhao, J.; Stenzel, M.H. Light-Induced Release of Molecules from Polymers. Prog. Polym. Sci. 2017, 74, 1–33. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Zhao, L.; Hou, X.; Liu, L.; Feng, B.; Li, M.; Zheng, P.; Zhao, X.; Wei, S. UV LED Curable Epoxy Soybean-Oil-Based Waterborne PUA Resin for Wood Coatings. Prog. Org. Coatings 2021, 151, 105942. [Google Scholar] [CrossRef]
- Li, C.; Xiao, H.; Wang, X.; Zhao, T. Development of Green Waterborne UV-Curable Vegetable Oil-Based Urethane Acrylate Pigment Prints Adhesive: Preparation and Application. J. Clean. Prod. 2018, 180, 272–279. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, H.; Hu, L.; Yang, Y.; Li, H.; Huang, C.; Liu, X. Novel Waterborne UV-Curable Hyperbranched Polyurethane Acrylate/Silica with Good Printability and Rheological Properties Applicable to Flexographic Ink. ACS Omega 2017, 2, 7546–7558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, W.; Sun, F. Synthesis and Properties of Waterborne UV-curable Polydimethylsiloxane-based Polyurethane Oligomers: UV-cured Film with Excellent Water Resistance and Thermostability. J. Adhes. Sci. Technol. 2020, 34, 2245–2261. [Google Scholar] [CrossRef]
- Fu, J.; Wang, L.; Yu, H.; Haroon, M.; Haq, F.; Shi, W.; Wu, B.; Wang, L. Research Progress of UV-Curable Polyurethane Acrylate-Based Hardening Coatings. Prog. Org. Coatings 2019, 131, 82–99. [Google Scholar] [CrossRef]
- Yin, W.; Zeng, X.; Li, H.; Hou, Y.; Gao, Q. Synthesis, Photopolymerization Kinetics, and Thermal Properties of UV-Curable Waterborne Hyperbranched Polyurethane Acrylate Dispersions. J. Coatings Technol. Res. 2011, 8, 577–584. [Google Scholar] [CrossRef]
- Chen, G.; Ouyang, S.; Deng, Y.; Chen, M.; Zhao, Y.; Zou, W.; Zhao, Q. Improvement of Self-Cleaning Waterborne Polyurethane-Acrylate with Cationic TiO2/Reduced Graphene Oxide. RSC Adv. 2019, 9, 18652–18662. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Jiang, Y.; Qiu, F.; Dai, Y.; Yang, D.; Yu, Z.; Yang, P. Synthesis, Mechanical Properties and Iron Surface Conservation Behavior of UV-Curable Waterborne Polyurethane-Acrylate Coating Modified with Inorganic Carbonate. Polym. Bull. 2018, 75, 4713–4734. [Google Scholar] [CrossRef]
- Shan, C.; Ning, C.; Lou, J.; Xu, W.; Zhang, Y. Design and Preparation of UV-Curable Waterborne Polyurethane Based on Novel Fluorinated Chain Extender. Polym. Bull. 2021, 78, 2067–2083. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; He, M.; Yang, H.; Hu, S.; Liao, Q. Preparation and Characterization of UV-Curable Fluorine-Silicon Block Urethane Acrylates for Application in Release Films. Prog. Org. Coatings 2019, 129, 171–177. [Google Scholar] [CrossRef]
- Gu, H.; Guo, J.; He, Q.; Tadakamalla, S.; Zhang, X.; Yan, X.; Huang, Y.; Colorado, H.A.; Wei, S.; Guo, Z. Flame-Retardant Epoxy Resin Nanocomposites Reinforced with Polyaniline-Stabilized Silica Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 7718–7728. [Google Scholar] [CrossRef]
- Sun, J.; Fang, H.; Wang, H.; Yang, S.; Xiao, S.; Ding, Y. Waterborne Epoxy-Modified Polyurethane-Acrylate Dispersions with Nano-Sized Core-Shell Structure Particles: Synthesis, Characterization, and Their Coating Film Properties. J. Polym. Eng. 2017, 37, 113–123. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Thermal and Mechanical Properties of Epoxy Acrylate/Methacrylates UV Cured Coatings. Prog. Org. Coatings 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate Terminated Castor Oil-Based Polyurethane Prepolymer: Synthesis and Characterization. Prog. Org. Coatings 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Bai, C.Y.; Zhang, X.Y.; Dai, J.B.; Li, W.H. A New UV Curable Waterborne Polyurethane: Effect of CC Content on the Film Properties. Prog. Org. Coatings 2006, 55, 291–295. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, X.; Lin, G.; Xi, L.; Yang, Y.; Lei, D.; Dong, H.; Su, J.; Cui, Y.; Liu, X. Preparation, Characterization and Application of UV-Curable Flexible Hyperbranched Polyurethane Acrylate. Polymers 2017, 9, 552. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, H.; Miao, Y.; Qiao, L.; Wang, X.; Wang, F. UV-Curable Waterborne Polyurethane from CO2-Polyol with High Hydrolysis Resistance. Polymer 2016, 100, 219–226. [Google Scholar] [CrossRef]
- Wei, D.; Huang, X.; Zeng, J.; Deng, S.; Xu, J. Facile Synthesis of a Castor Oil-Based Hyperbranched Acrylate Oligomer and Its Application in UV-Curable Coatings. J. Appl. Polym. Sci. 2020, 137, 1–12. [Google Scholar] [CrossRef]
- Gong, R.; Cao, H.; Zhang, H.; Qiao, L.; Wang, X. UV-Curable Cationic Waterborne Polyurethane from CO2-Polyol with Excellent Water Resistance. Polymer (Guildf). 2021, 218, 123536. [Google Scholar] [CrossRef]
- Pang, Y.; Shiraishi, A.; Keil, D.; Popov, S.; Strehmel, V.; Jiao, H.; Gutmann, J.S.; Zou, Y.; Strehmel, B. NIR-Sensitized Cationic and Hybrid Radical/Cationic Polymerization and Crosslinking. Angew. Chemie Int. Ed. 2021, 60, 1465–1473. [Google Scholar] [CrossRef]










| Samples No. | Polymer Composition (%) | DBC (mmol/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| IPDI | PCD | BDO | DMPA | EA | PET3A | TEA | ||
| WPU-EA-0 | 36.61 | 29.39 | 2.30 | 5.00 | 0.0 | 23.11 | 3.58 | 2.32 |
| WPU-EA-3 | 35.66 | 28.62 | 1.64 | 5.00 | 3.0 | 22.31 | 3.58 | 2.35 |
| WPU-EA-6 | 34.70 | 27.85 | 0.97 | 5.00 | 6.0 | 21.90 | 3.58 | 2.41 |
| WPU-EA-9 | 33.74 | 27.08 | 0.30 | 5.00 | 9.0 | 21.29 | 3.58 | 2.45 |
| Samples No. | EA (%) | Appearance | Centrifugal Stability | Thermal Stability | Storage Duration (Month) |
|---|---|---|---|---|---|
| WPU-EA-0 | 0.0 | Blue translucent | Stability | Stability | >12 |
| WPU-EA-3 | 3.0 | Blue translucent | Stability | Stability | >12 |
| WPU-EA-6 | 6.0 | Slight milky | Stability | Stability | >12 |
| WPU-EA-9 | 9.0 | Milky white | Stability | Sedimentation | None |
| Samples No. | Decomposition Stage | |||
|---|---|---|---|---|
| T5% (°C) | T10% (°C) | T50% (°C) | TMax% (°C) | |
| WPU-EA-0 | 188 | 261 | 356 | 326 |
| WPU-EA-3 | 200 | 258 | 363 | 433 |
| WPU-EA-6 | 197 | 255 | 367 | 432 |
| WPU-EA-9 | 196 | 252 | 380 | 434 |
| Samples No. | Hardness | Adhesion | Gloss/° | Water Absorption (%) |
|---|---|---|---|---|
| WPU-EA-0 | 2H | 4B | 91.2 | 14.2 |
| WPU-EA-3 | 3H | 4B | 92.5 | 12.3 |
| WPU-EA-6 | 4H | 5B | 93.4 | 11.2 |
| WPU-EA-9 | 4H | 5B | 93.6 | 10.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Wen, X.; Xu, R.; Liu, Z.; Xiang, H.; Li, Z.; Liu, X. Preparation and Properties of Novel Modified Waterborne Polyurethane Acrylate. Coatings 2022, 12, 1135. https://doi.org/10.3390/coatings12081135
Luo Q, Wen X, Xu R, Liu Z, Xiang H, Li Z, Liu X. Preparation and Properties of Novel Modified Waterborne Polyurethane Acrylate. Coatings. 2022; 12(8):1135. https://doi.org/10.3390/coatings12081135
Chicago/Turabian StyleLuo, Qinghong, Xinyu Wen, Ruijie Xu, Zhu Liu, Hongping Xiang, Zhiquan Li, and Xiaoxuan Liu. 2022. "Preparation and Properties of Novel Modified Waterborne Polyurethane Acrylate" Coatings 12, no. 8: 1135. https://doi.org/10.3390/coatings12081135
APA StyleLuo, Q., Wen, X., Xu, R., Liu, Z., Xiang, H., Li, Z., & Liu, X. (2022). Preparation and Properties of Novel Modified Waterborne Polyurethane Acrylate. Coatings, 12(8), 1135. https://doi.org/10.3390/coatings12081135