Lavender Essential Oil as Antibacterial Treatment for Packaging Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lavender EO
2.1.1. GC–MS Analysis
2.1.2. Antimicrobial Testing of the Essential Oil
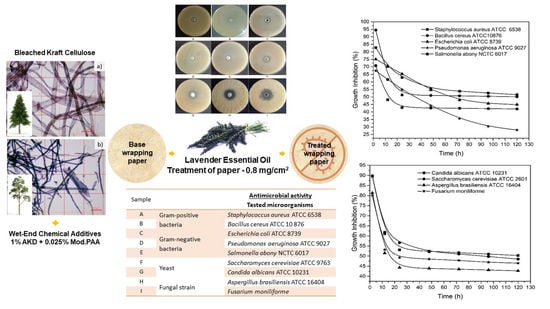
2.2. Paper Samples
2.2.1. Microscopic Analysis
2.2.2. The Papermaking Process
2.2.3. Basic Weight, Thickness, Density, Porosity, Smoothness
2.2.4. Tensile Strength, TEA Index, Elongation and Tear Resistance
2.3. Lavender EO Treatment of Obtained Wrapping Paper
2.4. Antimicrobial Testing of Treated Wrapping Paper
3. Results
3.1. Lavender EO Characteristics
3.2. Antimicrobial Activity of Lavender EO
3.3. Cellulose Fiber Characterization
3.4. Base Paper and Treated Paper Characterization
3.5. Antimicrobial Activity of Paper Treated with Lavender EO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Choi, J.; Ko, S. Applications of Nanomaterials in Food Packaging. J. Nanosci. Nanotechnol. 2015, 15, 6357–6372. [Google Scholar] [CrossRef] [PubMed]
- Dobre, L.M.; Stoica, A.; Stroescu, M.; Dobre, T.; Stefanov, S. New Biodegradable Composite Materials Based on Bacterial Cellulose for Food Packaging; NUHT: Kiev, Ukraine, 2011. [Google Scholar]
- Imran, M.; Revol-Junelles, A.-M.; Martyn, A.; Tehrany, E.A.; Jacquot, M.; Linder, M.; Desobry, S. Active Food Packaging Evolution: Transformation from Micro- to Nanotechnology. Crit. Rev. Food Sci. Nutr. 2010, 50, 799–821. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of Mechanical Properties, Migration, and Potential Applications in Active Food Packaging Systems Containing Nanoclays and Nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef] [Green Version]
- Realini, C.E.; Marcos, B. Active and Intelligent Packaging Systems for a Modern Society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef] [Green Version]
- Nerín, C.; Tovar, L.; Djenane, D.; Camo, J.; Salafranca, J.; Beltrán, J.A.; Roncalés, P. Stabilization of Beef Meat by a New Active Packaging Containing Natural Antioxidants. J. Agric. Food Chem. 2006, 54, 7840–7846. [Google Scholar] [CrossRef]
- Preedy, V. Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of Essential Oils in Active Food Packaging: Recent Advances and Future Trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential Oils and Their Principal Constituents as Antimicrobial Agents for Synthetic Packaging Films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef] [Green Version]
- Predoi, D.; Iconaru, S.; Buton, N.; Badea, M.; Marutescu, L. Antimicrobial Activity of New Materials Based on Lavender and Basil Essential Oils and Hydroxyapatite. Nanomaterials 2018, 8, 291. [Google Scholar] [CrossRef]
- Dobre, L.M.; Stoica, A.; Stroescu, M.; Dobre, T.; Stefanov, S.; Denkova, Z.; Nikolova, R.; Hadzhiyski, V.; Hristov, H. New biobased antimicrobial food packaging materials. Ann. Dunarea Jos Univ. Galati Fascicle IX 2011, SI, 75–84. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of Lavender (Lavandula Angustifolia) and Melissa (Melissa Officinalis) Waste on Quality and Shelf Life of Bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of Chitosan Beads Incorporated with Lavender or Red Thyme Essential Oils in Inhibiting Botrytis Cinerea and Their Application in Strawberry Packaging System. LWT 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Hossain, S.; Heo, H.; De Silva, B.C.J.; Wimalasena, S.H.M.P.; Pathirana, H.N.K.S.; Heo, G.-J. Antibacterial Activity of Essential Oil from Lavender (Lavandula Angustifolia) against Pet Turtle-Borne Pathogenic Bacteria. Lab. Anim. Res. 2017, 33, 195. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, E.; Stoyanova, A. Directory of the Specialist in the Aromatic Industry; UFT Academic Publishing House: Plovdiv, Bulgaria, 2006. [Google Scholar]
- Stoyanova, A.; Balinova-Tcvetkova, A. Lavender: Obtaining Essential Oil Products in Bulgaria; UFT Academic Publishing House: Plovdiv, Bulgaria, 2019. [Google Scholar]
- Stoyanova, A. A Guide for the Specialist in the Aromatic Industry; Bulgarian National Association of Essential Oils, Perfumery and Cosmetics: Plovdiv, Bulgaria, 2022. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender Essential Oil: A Review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The Antibacterial Activity of Lavender Essential Oil Alone and In Combination with Octenidine Dihydrochloride against MRSA Strains. Molecules 2020, 25, 95. [Google Scholar] [CrossRef] [Green Version]
- de Rapper, S.; Viljoen, A.; van Vuuren, S. The In Vitro Antimicrobial Effects of Lavandula Angustifolia Essential Oil in Combination with Conventional Antimicrobial Agents. Evid. Based Complement. Altern. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef] [Green Version]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Rungwasantisuk, A.; Raibhu, S. Application of Encapsulating Lavender Essential Oil in Gelatin/Gum-Arabic Complex Coacervate and Varnish Screen-Printing in Making Fragrant Gift-Wrapping Paper. Prog. Org. Coat. 2020, 149, 105924. [Google Scholar] [CrossRef]
- Stoyanova, А.; Georgiev, E.; Atanasova, T. Laboratory Manual for Essential Oils; UFT Academic Publishing House: Plovdiv, Bulgaria, 2007. [Google Scholar]
- Stankov, S.; Fidan, H.; Stefanova, G.; Kostova, I.; Damyanova, S.; Dimitrova-Dyulgerova, I.; Ercisli, S.; Stoyanova, A. Chemical Composition and Antimicrobial Activity of Essential Oil from Aerial Part (Leaves and Fruit) of Eucalyptus Gomphocephala DC. J. Essent. Oil Bear. Plants 2020, 23, 204–212. [Google Scholar] [CrossRef]
- Hussein, K.N.; Molnár, T.; Pinter, R.; Toth, A.; Ayari, E.; Friedrich, L.; Dalmadi, I.; Kiskó, G. In Vitro Antimicrobial Activity of Plant Active Components against Pseudomonas Lundensis and Listeria Monocytogenes. Progress 2021, 16, 163–172. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todorova, D.; Dimitrov, K.; Herzog, M. Solvent free UV curable coating for paper protection. Sustain. Chem. Pharm. 2021, 24, 100543. [Google Scholar] [CrossRef]
- Todorova, D.; Yavorov, N.; Lasheva, V. Improvement of Barrier Properties for Packaging Applications. Sustain. Chem. Pharm. 2022, 27, 100685. [Google Scholar] [CrossRef]
- Miladinova, P.M.; Todorova, D.A. Synthesis, Characterization, and Application of New Reactive Triazine Dye on Cotton and Paper. Fibers Polym. 2022, 23, 1614–1620. [Google Scholar] [CrossRef]
- Rodríguez, A.; Batlle, R.; Nerín, C. The use of natural essential oils as antimicrobial solutions in paper packaging. Part II. Prog. Org. Coat. 2007, 60, 33–38. [Google Scholar] [CrossRef]
- Rodríguez, A.; Nerín, C.; Batlle, R. New cinnamon-based active paper packaging against Rhizopus stolonifer food spoilage. J. Agric. Food Chem. 2008, 56, 6364–6369. [Google Scholar] [CrossRef]
- Perovic, S.; Pantovic, S.; Scepanovic, V.; Perovic, A.; Zivkovic, V.; Zivkovic, V.; Damjanovic Vratnica, B. Evaluation of Antimicrobial Activity and Activity on the Autonomic Nervous System of the Lavender Essential Oils from Montenegro. Prog. Nutr. 2019, 21, 584–590. [Google Scholar] [CrossRef]




| Characteristics | Lavender EO |
|---|---|
| Appearance | Transparent, very mobile liquid |
| Color | Colorless to pale yellow |
| Odor | Floral herbaceous scent and balsamic—woody undertone |
| Relative density, d | 0.8862 ± 0.05 |
| Refractive index, n | 1.4626 ± 0.02 |
| Polarization coefficient, α | −8 ± 0.02 |
| Acid number, (mg KOH/g oil) | 0.9 ± 0.04 |
| № | Ingredients | RT | Content, % |
|---|---|---|---|
| 1. | α-Thujene | 9.70 | 0.15 |
| 2. | α-Pinene | 9.93 | 0.31 |
| 3. | Camphene | 10.47 | 0.23 |
| 4. | β-Pinene | 11.60 | 0.19 |
| 5. | 3-Octanone | 11.74 | 0.22 |
| 6. | Myrcene | 11.81 | 0.53 |
| 7. | δ 3-carene | 12.42 | 0.11 |
| 8. | Hexyl acetate | 12.62 | 0.07 |
| 9. | p-Cymene | 13.10 | 0.57 |
| 10. | Limonene | 13.23 | 0.29 |
| 11. | Eucalyptol + β-Phellandrene | 13.27 | 0.68 |
| 12. | β-cis-Ocimene | 13.35 | 5.52 |
| 13. | β-trans-Ocimene | 13.67 | 1.79 |
| 14. | cis-Linalyl Oxide | 14.54 | 0.18 |
| 15. | trans-Linalyl Oxide | 15.05 | 0.10 |
| 16. | β-Linalool | 15.55 | 27.67 |
| 17. | 1-Octen-3-yl-acetate | 15.67 | 1.28 |
| 18. | Camphor | 16.90 | 0.22 |
| 19. | Lavandulol | 17.40 | 0.70 |
| 20. | Borneol | 17.67 | 0.66 |
| 21. | Terpinene-4-ol | 17.94 | 6.13 |
| 22. | Cryptone | 18.12 | 0.10 |
| 23. | Hexyl isobutyrate | 18.18 | 0.11 |
| 24 | α-Terpineol | 18.35 | 1.72 |
| 25. | Nerol | 19.21 | 0.14 |
| 26. | Linalyl acetate | 19.96 | 36.05 |
| 27. | Lavandulyl acetate | 20.82 | 4.87 |
| 28. | Neryl acetate | 22.82 | 0.51 |
| 29. | Geranyl acetate | 23.35 | 0.97 |
| 30. | β-Caryophyllene | 24.50 | 4.68 |
| 31. | α-Bergamotene | 24.78 | 0.17 |
| 32. | (Z)-β-Farnesene | 25.25 | 1.89 |
| 33. | α-Caryophyllene | 25.40 | 0.16 |
| 34. | Germacrene D | 26.03 | 0.19 |
| 35. | Caryophyllene oxide | 28.52 | 0.53 |
| Total | 99.69 |
| Sample | Tested Microorganisms | Inhibition Zone (mm) | |
|---|---|---|---|
| A | Gram-positive bacteria | Staphylococcus aureus | 13.9 ± 0.2 |
| B | Bacillus cereus | 14.5 ± 0.4 | |
| C | Gram-negative bacteria | Escherichia coli | 12.4 ± 0.1 |
| D | Pseudomonas aeruginosa | 11.7 ± 0.5 | |
| E | Salmonella abony | 11.1 ± 0.1 | |
| F | Yeast | Saccharomyces cerevisiae | 21.0 ± 0.2 |
| G | Candida albicans | 18.6 ± 0.3 | |
| H | Fungal strain | Aspergillus brasiliensis | 17.0 ± 0.5 |
| I | Fusarium moniliforme | 15.0 ± 0.5 |
| Paper Properties | Testing Method | Only Pulp | Base Paper | Lavender-Treated Paper | |
|---|---|---|---|---|---|
| Basic weight | ISO 536:2012 | g/m2 | 50.89 | 50.96 | 50.88 |
| Thickness | ISO 534:2011 | mm | 0.8 | 0.8 | 0.8 |
| Density | ISO 534:2011 | kg/m3 | 63.61 | 63.73 | 63.42 |
| Porosity | ISO 534:2011 | % | 95.76 | 95.75 | 96.76 |
| Smoothness (Bekk, top side) | ISO 5627/A1:2004 | s | 10.98 | 10.96 | 10.89 |
| Sample | Composition | Tensile Index, Nm/g | TEA Index, mJ/g | Elongation, % | Tear Index, Mn·m2/g |
|---|---|---|---|---|---|
| 0 | Only pulp | 63.0 | 1070 | 2.4 | 1.1004 |
| 1 | Base paper | 69.5 | 1430 | 2.9 | 1.0989 |
| 2 | Lavender-treated paper | 65.6 | 1210 | 2.6 | 1.1084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, D.; Yavorov, N.; Lasheva, V.; Damyanova, S.; Kostova, I. Lavender Essential Oil as Antibacterial Treatment for Packaging Paper. Coatings 2023, 13, 32. https://doi.org/10.3390/coatings13010032
Todorova D, Yavorov N, Lasheva V, Damyanova S, Kostova I. Lavender Essential Oil as Antibacterial Treatment for Packaging Paper. Coatings. 2023; 13(1):32. https://doi.org/10.3390/coatings13010032
Chicago/Turabian StyleTodorova, Dimitrina, Nikolay Yavorov, Veska Lasheva, Stanka Damyanova, and Iliana Kostova. 2023. "Lavender Essential Oil as Antibacterial Treatment for Packaging Paper" Coatings 13, no. 1: 32. https://doi.org/10.3390/coatings13010032
APA StyleTodorova, D., Yavorov, N., Lasheva, V., Damyanova, S., & Kostova, I. (2023). Lavender Essential Oil as Antibacterial Treatment for Packaging Paper. Coatings, 13(1), 32. https://doi.org/10.3390/coatings13010032