A Review—Effect of Accelerating Methods on Gas Nitriding: Accelerating Mechanism, Nitriding Behavior, and Techno-Economic Analysis
Abstract
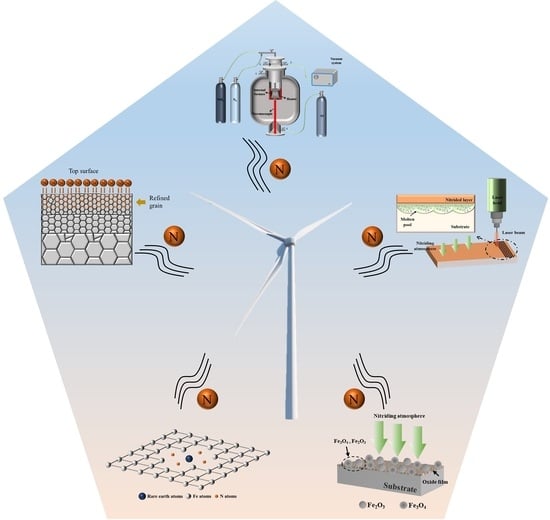
:1. Introduction
2. Research Progress of Conventional Gas Nitriding
2.1. Mechanism of Gas Nitriding
2.2. Nitrided Layer Structure of Gas Nitriding
2.3. Process Parameters of Gas Nitriding
3. Effect of Accelerating Nitriding Methods on the Behavior and Efficiency of Gas Nitriding
3.1. Process Parameter Optimization
3.1.1. Accelerating Nitriding Mechanism of Optimizing Process Parameters
3.1.2. Effect of Process Parameter Optimization on Nitriding Behavior
Effect of Pressure on Nitriding Behavior
Effect of Temperature on Nitriding Behavior
3.1.3. Effect of Process Parameter Optimization on Nitriding Efficiency
3.2. Surface Mechanical Nano-Crystallization
3.2.1. Accelerating the Nitriding Mechanism of Surface Mechanical Nano-Crystallization
3.2.2. Effect of Surface Mechanical Nano-Crystallization on Nitriding Behavior
3.2.3. Effect of Surface Mechanical Nano-Crystallization on Nitriding Efficiency
3.3. Surface-Active Catalytic Nitriding
3.3.1. Accelerating Nitriding Mechanism of Surface-Active Catalytic Nitriding
3.3.2. Effect of Surface-Active Catalytic on Nitriding Behavior
Effect of Alloying Elements (Ni, C, Ti, B, etc.) on Nitriding Behavior
Effect of Rare-Earth Elements (Re) on Nitriding Behavior
3.3.3. Effect of Surface-Active Catalytic Nitriding on Nitriding Efficiency
3.4. Surface Pre-Oxidized Nitriding
3.4.1. Accelerating Nitriding Mechanism of Surface Pre-Oxidized Nitriding
3.4.2. Effect of Surface Pre-Oxidation on Nitriding Behavior
3.4.3. Effect of Surface Pre-Oxidation on Nitriding Efficiency
3.5. Surface Laser Treatment
3.5.1. Accelerating Nitriding Mechanism of Surface Laser Treatment
3.5.2. Effect of Surface Laser Treatment on Nitriding Behavior
3.5.3. Effect of Surface Laser Treatment on Nitriding Efficiency
4. Comparison of Technical Economy and Technology Readiness Level of Various Infiltration Methods
4.1. Technical Economy
| Type | Energy Consumption (kWh/kg) | CO2 Emissions (kg) | Indicative CAPEX (USD/year) | Indicative OPEX (USD/year) | Ref. |
|---|---|---|---|---|---|
| Conventional gas nitriding | 35–45 | 6.5–7.5 | 300–350 | 40–45 | [34,48,107,137] |
| Process parameter optimization | 45–50 | 7.5–8.0 | 250–300 | 30–35 | [22,71,98,100] |
| Surface mechanical nano-crystallization | 15–20 | 4.5–5.5 | 450–500 | 55–60 | [32,41,96,102] |
| Surface-active catalytic nitriding | 12–15 | 3.5–4.5 | 750–800 | 70–75 | [53,62,64,158] |
| Surface pre-oxidation | 10–12 | 3–4 | 850–900 | 90–95 | [75,127,169,180] |
| Surface laser treatment | 8–10 | 2.5–3.5 | 1100–1150 | 100–105 | [24,184,195,196] |
4.2. Technology Readiness Level
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendoza, J.M.F.; Ibarra, D. Technology-enabled circular business models for the hybridisation of wind farms: Integrated wind and solar energy, power-to-gas and power-to-liquid systems. Sustain. Prod. Consum. 2023, 36, 308–327. [Google Scholar] [CrossRef]
- Watson, S.; Moro, A.; Reis, V.; Baniotopoulos, C.; Barth, S.; Bartoli, G.; Bauer, F.; Boelman, E.; Bosse, D.; Cherubini, A.; et al. Future emerging technologies in the wind power sector: A European perspective. Renew. Sustain. Energy Rev. 2019, 113, 109270. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X. Wind power forecasting of an offshore wind turbine based on high-frequency SCADA data and deep learning neural network. Energy 2020, 201, 117693. [Google Scholar] [CrossRef]
- Bharani, R.; Sivaprakasam, A. A meteorological data set and wind power density from selective locations of Tamil Nadu, India: Implication for installation of wind turbines. Total Environ. Res. Themes 2022, 3–4, 100017. [Google Scholar] [CrossRef]
- Wang, H.; Lamichhane, T.N.; Paranthaman, M.P. Review of additive manufacturing of permanent magnets for electrical machines: A prospective on wind turbine. Mater. Today Phys. 2022, 24, 100675. [Google Scholar] [CrossRef]
- Pogrennjak, A.D.; Bratushka, S.N.; Bersnev, V.M.; Levintant-Zayonts, N. Shape memory effect and superelasticity of titanium nickelide alloys implanted with high ion doses. Russ. Chem. Rev. 2013, 82, 1135–1159. [Google Scholar] [CrossRef]
- Qian, H.; Chen, S.; Wang, T.; Cheng, G.; Chen, X.; Xu, Z.W.; Zeng, Q.; Liu, Y.; Yan, D.M. Silicon nitride modified enamel coatings enable high thermal shock and corrosion resistances for steel protection. Surf. Coat. Technol. 2021, 421, 127474. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H. Development of offshore wind power and foundation technology for offshore wind turbines in China. Ocean Eng. 2022, 266, 113256. [Google Scholar] [CrossRef]
- Dhanola, A.; Garg, H.C. Tribological challenges and advancements in wind turbine bearings: A review. Eng. Fail. Anal. 2020, 118, 104885. [Google Scholar] [CrossRef]
- Nassar, N.T.; Wilburn, D.R.; Goonan, T.G. Byproduct metal requirements for US wind and solar photovoltaic electricity generation up to the year 2040 under various Clean Power Plan scenarios. Appl. Energy 2016, 183, 1209–1226. [Google Scholar] [CrossRef]
- Vallejo, J.P.; Álvarez-Regueiro, E.; Cabaleiro, D.; Fernandez-Seara, J.; Fernandez, J.; Lugo, L. Functionalized graphene nanoplatelet nanofluids based on a commercial industrial antifreeze for the thermal performance enhancement of wind turbines. Appl. Therm. Eng. 2019, 152, 113–125. [Google Scholar] [CrossRef]
- Yan, F.Y.; Chen, B.F.; Yao, J.W.; Zhang, D.L.; Yan, M.F.; Zhang, Y.X. Characterization of microstructure and corrosion properties of AZ91D magnesium alloy surface-treated by coating-nitriding. J. Mater. Res. Technol. 2021, 14, 1559–1568. [Google Scholar] [CrossRef]
- Pohreliuk, I.M.; Yaskiv, O.I.; Fedirko, V.M.; Diuh, I.V. Effect of nitriding on the electrochemical behaviour of Ti-Al-Mn alloy. Mater. Corros. 2005, 56, 697–700. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and corrosion resistance of plasma nitrided stainless steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- Peng, D.Q.; Kim, T.H.; Chung, J.H.; Park, J.K. Development of nitride-layer of AISI 304 austenitic stainless steel during high-temperature ammonia gas-nitriding. Appl. Surf. Sci. 2010, 256, 7522–7529. [Google Scholar] [CrossRef]
- Dong, H. S-phase surface engineering of Fe-Cr, Co-Cr and Ni-Cr alloys. Int. Mater. Rev. 2010, 55, 65–98. [Google Scholar] [CrossRef]
- Michalski, J.; Wach, P.; Tacikowski, J.; Betiuk, M.; Burdynski, K.; Kowalski, S.; Nakonieczny, A. Contemporary industrial application of nitriding and its modifications. Mater. Manuf. Process. 2009, 24, 855–858. [Google Scholar] [CrossRef]
- Almeida, E.A.S.; Costa, C.E.; Milan, J.C.G. Study of the nitrided layer obtained by different nitriding methods. Matéria 2015, 20, 460–465. [Google Scholar] [CrossRef]
- Tong, W.P.; Liu, C.Z.; Wang, W.; Tao, N.R.; Wang, Z.B.; Zuo, L.; He, J.C. Gaseous nitriding of iron with a nanostructured surface layer. Scr. Mater. 2007, 57, 533–536. [Google Scholar] [CrossRef]
- Wang, B.; Fu, W.T.; Dong, F.; Jin, G.F.; Feng, W.W.; Wang, Z.H.; Sun, S.H. Significant acceleration of nitriding kinetics in pure iron by pressurized gas treatment. Mater. Des. 2015, 85, 91–96. [Google Scholar] [CrossRef]
- Keddam, M.; Djeghlal, M.E.; Barrallier, L. A diffusion model for simulation of bilayer growth (ε/γ′) of nitrided pure iron. Mater. Sci. Eng. 2004, 378, 475–478. [Google Scholar] [CrossRef]
- Alekseeva, M.S.; Gress, M.A.; Scherbakov, S.P.; Gerasimov, S.A.; Kuksenova, L.I. The influence of high-pressure gas nitriding on the properties of martensitic steels. Met. Sci. Heat Treat. 2017, 59, 524–528. [Google Scholar] [CrossRef]
- Singh, S.K.; Naveen, C.; Sai, Y.V.; Satish, U.; Bandhavi, C.; Subbiah, R. Experimental study on wear resistance of AISI 347 treated with salt bath nitriding and gas nitriding processes-a review. Mater. Today Proc. 2019, 18, 2717–2722. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.G. Effect of Gas Nitriding Characteristics on the Mechanical Properties after Pre-Heat Treatment of Stainless Steels. J. Korean Soc. Heat Treat. 2010, 23, 142–149. [Google Scholar]
- Boztepe, E.; Alves, A.C.; Ariza, E.; Rocha, L.A.; Cansever, N.; Toptan, F. A comparative investigation of the corrosion and tribocorrosion behaviour of nitrocarburized, gas nitrided, fluidized-bed nitrided, and plasma nitrided plastic mould steel. Surf. Coat. Technol. 2018, 334, 116–123. [Google Scholar] [CrossRef]
- Ichii, K. Structure of the ion-nitrided layer of 18-8 stainless steel. Technol. Rep. Kansai Univ. 1986, 27, 135. [Google Scholar]
- Kundalkar, D.; Mavalankar, M.; Tewari, A. Effect of gas nitriding on the thermal fatigue behavior of martensitic chromium hot-work tool steel. Mater. Sci. Eng. 2016, 651, 391–398. [Google Scholar] [CrossRef]
- Pellizzari, M.; Molinari, A.; Straffelini, G. Thermal fatigue resistance of gas and plasma nitrided 41CrAlMo7 steel. Mater. Sci. Eng. 2003, 352, 186–194. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Arif, A.F.M.; Yilbas, B.S. Evaluation of gas nitriding process with in-process variation of nitriding potential for AISI H13 tool steel. Int. J. Adv. Manuf. Technol. 2010, 47, 687–698. [Google Scholar] [CrossRef]
- Moradshahi, M.; Tavakoli, T.; Amiri, S.; Shayeganmehr, S. Plasma nitriding of Al alloys by DC glow discharge. Surf. Coat. Technol. 2006, 201, 567–574. [Google Scholar] [CrossRef]
- Funch, C.V.; Christiansen, T.L.; Somers, M.A.J. Gaseous nitriding of additively manufactured maraging steel; nitriding kinetics and microstructure evolution. Surf. Coat. Technol. 2022, 432, 128055. [Google Scholar] [CrossRef]
- Li, G.M.; Liang, Y.L.; Sun, H.; Cao, Y.G. Nitriding behavior and mechanical properties of carburizing and nitriding duplex treated M50NiL steel. Surf. Coat. Technol. 2020, 384, 125315. [Google Scholar] [CrossRef]
- Baranowska, J.; Arnold, B. Corrosion resistance of nitrided layers on austenitic steel. Surf. Coat. Technol. 2006, 200, 6623–6628. [Google Scholar] [CrossRef]
- Somers, M.A.J.; Mittemeijer, E.J. Layer-growth kinetics on gaseous nitriding of pure iron: Evaluation of diffusion coefficients for nitrogen in iron nitrides. Metall. Mater. Trans. A 1995, 26, 57–74. [Google Scholar] [CrossRef]
- Yang, S.; Yang, D.; Shi, W.; Deng, C.; Chen, C.; Feng, S. Global evaluation of carbon neutrality and peak carbon dioxide emissions: Current challenges and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 81725–81744. [Google Scholar] [CrossRef] [PubMed]
- Karakan, M.; Alsaran, A.; Celik, A. Effect of process time on structural and tribological properties of ferritic plasma nitrocarburized AISI 4140 steel. Mater. Des. 2004, 25, 349–353. [Google Scholar] [CrossRef]
- Michalski, J.; Tacikowski, J.; Wach, P.; Ratajski, J. Controlled gas nitriding of 40HM and 38HMJ steel grades with the formation of nitrided cases with and without the surface compound layer, composed of iron nitrides. Probl. Eksploat. 2006, 2, 43–52. [Google Scholar]
- Fattah, M.; Mahboubi, F. Comparison of ferritic and austenitic plasma nitriding and nitrocarburizing behavior of AISI 4140 low alloy steel. Mater. Des. 2010, 31, 3915–3921. [Google Scholar] [CrossRef]
- Kumar, S.A.; Raman, S.G.S.; Narayanan, T.S.N.S.; Gnanamoorthy, R. Influence of counterbody material on fretting wear behaviour of surface mechanical attrition treated Ti–6Al–4V. Tribol. Int. 2013, 57, 107–114. [Google Scholar] [CrossRef]
- Liu, B.; Wang, B.; Yang, X.D.; Zhao, X.F.; Qin, M.; Gu, J.F. Thermal fatigue evaluation of AISI H13 steels surface modified by gas nitriding with pre-and post-shot peening. Appl. Surf. Sci. 2019, 483, 45–51. [Google Scholar] [CrossRef]
- Liu, J.; Suslov, S.; Vellore, A.; Ren, Z.C.; Amanov, A.; Pyun, Y.S.; Martini, A.; Dong, Y.L.; Ye, C. Surface nanocrystallization by ultrasonic nano-crystal surface modification and its effect on gas nitriding of Ti6Al4V alloy. Mater. Sci. Eng. 2018, 736, 335–343. [Google Scholar] [CrossRef]
- Rodrigues, J.; Miranda, S.M.C.; Santos, N.F.; Neves, A.J.; Alves, E.; Lorenz, K.; Monteiro, T. Rare earth co-doping nitride layers for visible light. Mater. Chem. Phys. 2012, 134, 716–720. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Chen, X.; Chen, H.T.; Wu, Y.Q.; Wang, Y.X.; Tang, L.N.; Cui, G.D.; Chen, D.Z. Catalytic behavior of LaFeO3 pervoskite oxide during low-pressure gas nitriding. Appl. Surf. Sci. 2020, 506, 145045. [Google Scholar] [CrossRef]
- Zhang, C.S.; Yan, M.F.; Sun, Z. Experimental and theoretical study on interaction between lanthanum and nitrogen during plasma rare earth nitriding. Appl. Surf. Sci. 2013, 287, 381–388. [Google Scholar] [CrossRef]
- Sueyoshi, H.; Hamaishi, K.; Kadomatsu, S.; Shiomizu, T.; Ohzono, Y. Effect of preheating in air on gas nitriding of SUS304. Nippon Kinzoku Gakkaishi (1952) 1996, 60, 616–623. [Google Scholar]
- Lutz, J.; Mändl, S. Effect of ion energy and chemistry on layer growth processes during nitriding of CoCr alloys. Nucl. Instrum. Methods Phys. Res. Sect. B 2009, 267, 1522–1525. [Google Scholar] [CrossRef]
- Li, S.X.; Chen, L.; Wang, M.T.; Yan, Z.Q. Effect of pre-oxidation and rare earth cerium on plasma nitriding of 42CrMo steel. Heat Treat. Met. 2021, 46, 186–189. [Google Scholar]
- Schaaf, P. Laser nitriding of metals. Prog. Mater. Sci. 2002, 47, 1–161. [Google Scholar] [CrossRef]
- Chen, X.K.; Wu, G.; Wang, R.; Guo, W.T.; Yang, J.P.; Cao, S.Z.; Wang, Y.L.; Han, W.H. Laser nitriding of titanium alloy in the atmosphere environment. Surf. Coat. Technol. 2007, 201, 4843–4846. [Google Scholar] [CrossRef]
- Razavi, R.S.; Gordani, G.R.; Man, H.C. A review of the corrosion of laser nitrided Ti-6Al-4V. Anti-Corros. Methods Mater. 2011, 58, 140–154. [Google Scholar] [CrossRef]
- Fang, B.Z.; Daniel, L.; Bonakdarpour, A.; Wilkinson, D.P. Upgrading the State-of-the-Art Electrocatalysts for Proton Exchange Membrane Fuel Cell Applications. Adv. Mater. Interfaces 2022, 9, 2200349. [Google Scholar] [CrossRef]
- Zhao, M.M.; Huang, X.L.; Zhuang, D.M.; Sheng, L.; Xie, X.; Cao, M.; Pan, J.J.; Fan, H.Y.; He, J.P. Constructing porous nanosphere structure current collector by nitriding for lithium metal batteries. J. Energy Storage 2022, 47, 103665. [Google Scholar] [CrossRef]
- Arabczyk, W.; Skulmowska, K.; Pelka, R.; Lendzion-Bielun, Z. Oscillatory Mechanism of α-Fe (N)↔ γ’-Fe4N Phase Transformations during Nanocrystalline Iron Nitriding. Materials 2022, 15, 1006. [Google Scholar] [CrossRef]
- Sun, J.Q.; Wang, D.R.; Yang, J.; Li, F.J.; Zuo, L.L.; Ge, F.; Chen, Y.B. In Situ Preparation of Nano-Cu/Microalloyed Gradient Coating with Improved Antifriction Properties. Coatings 2022, 12, 1336. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, S.; Zheng, J.; Liang, L. Advances in Latest Application Status, Challenges, and Future Development Direction of Electrospinning Technology in the Biomedical. J. Nanomater. 2022, 2022, 3791908. [Google Scholar] [CrossRef]
- Xu, W.C.; Cui, Z.D.; Zhu, S.L. Recent Advances in Open-Cell Porous Metal Materials for Electrocatalytic and Biomedical Applications. Acta Metall. Sin. 2022, 58, 1527–1544. [Google Scholar]
- Bell, T. Source Book on Nitriding; American Society for Metals: Metals Park, OH, USA, 1977. [Google Scholar]
- García Caballero, F.; Wang Fu, M.; Gao, M.C. Encyclopedia of Materials: Metals and Alloys; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chen, W.L.; Wu, C.L.; Liu, Z.R.; Ni, S.; Hong, Y.; Zhang, Y.; Chen, J.H. Phase transformations in the nitrocarburizing surface of carbon steels revisited by microstructure and property characterizations. Acta Mater. 2013, 61, 3963–3972. [Google Scholar] [CrossRef]
- Wu, C.L.; Tian, L.; Hong, Y.; Wang, J.; Chen, X.Y. The effect of cooling methods and subsequent ageing on the nitrided layer of pure-iron by gas nitriding at 580 °C. J. Hunan Univ. 2015, 42, 33–39. [Google Scholar]
- Wu, C.L.; Luo, C.P.; Zou, G.F. Phase Transformations During Composite-Chromization of steel 20. Acta Metall. Sin. 2004, 40, 1074–1078. [Google Scholar]
- Lin, Y.H.; Bohn, A.; Cheng, J.Y.; Handt, A.V.D.; Mara, N.A.; Poerschke, D. Gas nitriding behavior of refractory metals and implications for multi-principal element alloy design. J. Alloys Compd. 2023, 947, 169568. [Google Scholar] [CrossRef]
- Xie, Y.; Miyamoto, G.; Furuhara, T. High-throughput investigation of Cr-N cluster formation in Fe-35Ni-Cr system during low-temperature nitriding. Acta Mater. 2023, 253, 118921. [Google Scholar] [CrossRef]
- Fare, S.; Lecis, N.; Brescia, E.; Mazzola, M. Role of grain boundaries in diffusional phenomena during gas nitriding of pure iron. Procedia Eng. 2011, 10, 2943–2948. [Google Scholar] [CrossRef]
- Dupuis, R.D. Epitaxial growth of III–V nitride semiconductors by metalorganic chemical vapor deposition. J. Cryst. Growth 1997, 178, 56–73. [Google Scholar] [CrossRef]
- Jiang, X.J.; Wang, S.Z.; Feng, Z.H.; Qi, H.B.; Fu, H.; Liu, R.P. Improving vacuum gas nitriding of a Ti-based alloy via surface solid phase transformation. Vacuum 2022, 197, 110860. [Google Scholar] [CrossRef]
- Wang, J.; Shu, R.; Chai, J.L.; Rao, S.G.; Febvrier, A.L.; Wu, H.C.; Zhu, Y.B.; Yao, C.F.; Luo, L.H.; Li, W.P.; et al. Xe-ion-irradiation-induced structural transitions and elemental diffusion in high-entropy alloy and nitride thin-film multilayers. Mater. Des. 2022, 219, 110749. [Google Scholar] [CrossRef]
- Dossett, J.L.; Totten, G.E. Introduction to steel heat treatment. Steel Heat Treat. Fundam. Process. 2013, 4, 3–25. [Google Scholar]
- Bell, T.; Mao, K.; Sun, Y. Surface engineering design: Modelling surface engineering systems for improved tribological performance. Surf. Coat. Technol. 1998, 108, 360–368. [Google Scholar] [CrossRef]
- Gao, H.; Lysevych, M.; Tan, H.H.; Jagadish, C.; Zou, J. The effect of Sn addition on GaAs nanowire grown by vapor–liquid–solid growth mechanism. Nanotechnology 2018, 29, 465601. [Google Scholar] [CrossRef]
- Wang, B.; Sun, S.H.; Guo, M.W.; Jin, G.F.; Zhou, Z.A.; Fu, W.T. Study on pressurized gas nitriding characteristics for steel 38CrMoAlA. Surf. Coat. Technol. 2015, 279, 60–64. [Google Scholar] [CrossRef]
- Zhao, H.; Duan, L.; Chen, G.; Fan, H.Y.; Wang, J.; Zhou, C.C. High corrosion resistance performance of 304 stainless steel after liquid nitrocarburization. Compos. Part B 2018, 155, 173–177. [Google Scholar] [CrossRef]
- Mirjani, M.; Mazrooei, J.; Karimzadeh, N.; Ashrafizadeh, F. Investigation of the effects of time and temperature of oxidation on corrosion behavior of plasma nitrided AISI 4140 steel. Surf. Coat. Technol. 2012, 206, 4389–4393. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, D.D.; Shen, L. The effect of surface nanocrystallization on plasma nitriding behaviour of AISI 4140 steel. Appl. Surf. Sci. 2010, 257, 979–984. [Google Scholar] [CrossRef]
- Chen, X.; Bao, X.Y.; Xiao, Y.; Zhang, C.S.; Tang, L.N.; Yao, L.; Cui, G.D.; Yang, Y. Low-temperature gas nitriding of AISI 4140 steel accelerated by LaFeO3 perovskite oxide. Appl. Surf. Sci. 2019, 466, 989–999. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.L.; Shen, C.; Zhang, Y.L.; Li, F.; Ding, Y.H.; Xin, J.W.; Wang, B.S.; Hua, X.M. Effect of substrate temperature on microstructure and mechanical properties of TiAl alloy fabricated using the twin-wire plasma arc additive manufacturing system. J. Mater. Sci. 2022, 57, 8940–8955. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Fossati, A.; Bacci, T. Glow-discharge nitriding and post-oxidising treatments of AISI H11 steel. Surf. Coat. Technol. 2003, 162, 61–66. [Google Scholar] [CrossRef]
- Xiang, J.; Han, Y.F.; Le, J.W.; Huang, G.F.; Xiao, L.; Liu, J.Y.; Lu, W.J. Effect of temperature on microstructure and mechanical properties of ECAPed (TiB+La2O3)/Ti-6Al-4V composites. Mater. Charact. 2018, 146, 149–158. [Google Scholar] [CrossRef]
- Miyamoto, J.; Abraha, P. The effect of plasma nitriding treatment time on the tribological properties of the AISI H13 tool steel. Surf. Coat. Technol. 2019, 375, 15–21. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.H.; Yan, J.; Zen, D.Z.; Zhang, Q.A.; Huang, R.B.; Fan, H.Y. Influence of time on the microstructure of AISI 321 austenitic stainless steel in salt bath nitriding. Surf. Coat. Technol. 2012, 206, 3399–3404. [Google Scholar] [CrossRef]
- Arabczyk, W.; Zamlynny, J. Study of the ammonia decomposition over iron catalysts. Catal. Lett. 1999, 60, 167–171. [Google Scholar] [CrossRef]
- Wolowiec-Korecka, E.; Michalski, J.; Kucharska, B. Kinetic aspects of low-pressure nitriding process. Vacuum 2018, 155, 292–299. [Google Scholar] [CrossRef]
- Michalski, J.; Burdyński, K.; Wach, P.; Latas, Z. Nitrogen availability of nitriding atmosphere in controlled gas nitriding processes. Arch. Metall. Mater. 2015, 60, 747–754. [Google Scholar] [CrossRef]
- Abboud, J.H.; Fidel, A.F.; Benyounis, K.Y. Surface nitriding of Ti–6Al–4V alloy with a high power CO2 laser. Opt. Laser Technol. 2008, 40, 405–414. [Google Scholar] [CrossRef]
- Michalski, J.; Wołowiec-Korecka, E. A study of parameters of nitriding processes. Part 1. Met. Sci. Heat Treat. 2019, 61, 183–190. [Google Scholar] [CrossRef]
- Mitsui, H.; Kurihana, S. Solution nitriding treatment of Fe–Cr alloys under pressurized nitrogen gas. ISIJ Int. 2007, 47, 479–485. [Google Scholar] [CrossRef]
- Lehrer, E. Über das Eisen-Wasserstoff-Ammoniak-Gleichgewicht. Z. Elektrochem. Angew. Phys. Chem. 1930, 36, 383–392. [Google Scholar]
- Michalski, J.; Tacikowski, J.; Wach, P.; Lunarska, E.; Baum, H. Formation of single-phase layer of γ′-nitride in controlled gas nitriding. Met. Sci. Heat Treat. 2005, 47, 516–519. [Google Scholar] [CrossRef]
- Nakonieczny, A.; Senatorski, J.; Tacikowski, J.; Tymowski, G.; Liliental, W. Computer Controlled Gas Nitriding—A Viable Replacement for Carburising. Heat Treat. Met. 1998, 3, 46–51. [Google Scholar]
- Panfil, D.; Kulka, M.; Wach, P.; Michalski, J.; Przestacki, D. Nanomechanical properties of iron nitrides produced on 42CrMo4 steel by controlled gas nitriding and laser heat treatment. J. Alloys Compd. 2017, 706, 63–75. [Google Scholar] [CrossRef]
- Michalski, J. Characteristics and Calculations of Atmospheres for Controlled Gas Nitriding of Steel; Institute of Precision Mechanics: Warsaw, Poland, 2010. [Google Scholar]
- Widi, K.A.; Wardana, W.; Suprapto, W.; Irawan, Y. White layer control on AISI 316L using temperature and gas nitriding diffusion stage process. Int. Rev. Mech. Eng. 2017, 11, 613–618. [Google Scholar] [CrossRef]
- Yazıcı, M.; Çomaklı, O.; Yetim, T.; Yetim, A.F.; Celik, A. The effect of plasma nitriding temperature on the electrochemical and semiconducting properties of thin passive films formed on 316 L stainless steel implant material in SBF solution. Surf. Coat. Technol. 2015, 261, 181–188. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, D.; Han, D. Improvement of corrosion and wear resistances of AISI 420 martensitic stainless steel using plasma nitriding at low temperature. Surf. Coat. Technol. 2008, 202, 2577–2583. [Google Scholar] [CrossRef]
- Takesue, S.; Kikuchi, S.; Misaka, Y.; Morita, T.; Komotori, J. Rapid nitriding mechanism of titanium alloy by gas blow induction heating. Surf. Coat. Technol. 2020, 399, 126160. [Google Scholar] [CrossRef]
- Abrasonis, G.; Rivière, J.P.; Templier, C.; Muzard, S.; Pranevicius, L. Influence of surface preparation and ion flux on the nitriding efficiency of austenitic stainless steel. Surf. Coat. Technol. 2005, 196, 279–283. [Google Scholar] [CrossRef]
- Baranowska, J. Importance of surface activation for nitrided layer formation on austenitic stainless steel. Surf. Eng. 2010, 26, 293–298. [Google Scholar] [CrossRef]
- Priest, J.M.; Baldwin, M.J.; Fewell, M.P. The action of hydrogen in low-pressure rf-plasma nitriding. Surf. Coat. Technol. 2001, 145, 152–163. [Google Scholar] [CrossRef]
- Lu, S.J.; Zhao, X.B.; Wang, S.K.; Li, J.C.; Wei, W.; Hu, J. Performance enhancement by plasma nitriding at low gas pressure for 304 austenitic stainless steel. Vacuum 2017, 145, 334–339. [Google Scholar] [CrossRef]
- Wen, K.; Zhang, C.; Gao, Y. Influence of gas pressure on the low-temperature plasma nitriding of surface-nanocrystallined TC4 titanium alloy. Surf. Coat. Technol. 2022, 436, 128327. [Google Scholar] [CrossRef]
- Avelar-Batista, J.C.; Spain, E.; Housden, J.; Matthews, A.; Fuentes, G.G. Plasma nitriding of Ti6Al4V alloy and AISI M2 steel substrates using DC glow discharges under a triode configuration. Surf. Coat. Technol. 2005, 200, 1954–1961. [Google Scholar] [CrossRef]
- Wang, B.Y.; Zhou, S.N.; Wang, J.J.; Zhao, B. Effect of Gas Nitriding on CO2 Corrosion for 35CrMo Steel After Surface Nanocrystallization. J. Nanosci. Nanotechnol. 2014, 14, 8079–8082. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Watanabe, Y.; Umezawa, O. Effect of Nitriding Temperature and Compositions on Diffusion Layer’s Hardness in Gas Nitrided Low Alloy Steel Containing Chromium. J. Jpn. Inst. Met. Mater. 2016, 80, 253–258. [Google Scholar] [CrossRef]
- Syla, N.; Aliaj, F.; Rama, M. Hardness curves for 31CrMoV9 steel after gas nitriding. Acta Phys. Pol. A 2017, 132, 484–486. [Google Scholar] [CrossRef]
- Godec, M.; Ruiz-Zepeda, F.; Podgornik, B.; Donik, C.; Kocijan, A.; Balantic, D.A.S.B. The influence of the plasma-nitriding temperature on the microstructure evolution and surface properties of additive-manufactured 18Ni300 maraging steel. Surf. Coat. Technol. 2022, 433, 128089. [Google Scholar] [CrossRef]
- Michla, J.R.J.; Ravikumar, B.; Prabhu, T.R.; Siengchin, S.; Kumar, M.A.; Rajini, N. Effect of nitriding on mechanical and microstructural properties of Direct Metal Laser Sintered 17-4PH stainless steel. J. Mater. Res. Technol. 2022, 19, 2810–2821. [Google Scholar] [CrossRef]
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Lu, J.; Lu, K. Nitriding iron at lower temperatures. Science 2003, 299, 686–688. [Google Scholar] [CrossRef]
- Lu, K.; Lu, J. Surface nanocrystallization (SNC) of metallic materials-presentation of the concept behind a new approach. J. Mater. Sci. Technol. 1999, 15, 193–197. [Google Scholar]
- Hu, G.; Sheng, G.M.; Han, J. Investigation and Application of Surface Self Nano-crystallization Induced by Severe Plastic Deformation. Mater. Rep. 2007, 21, 117–121. [Google Scholar]
- Azadmanjiri, J.; Berndt, C.C.; Kapoor, A.; Wen, C. Development of surface nano-crystallization in alloys by surface mechanical attrition treatment (SMAT). Crit. Rev. Solid State Mater. Sci. 2015, 40, 164–181. [Google Scholar] [CrossRef]
- Bahl, S.; Suwas, S.; Ungar, T.; Chatterjee, K. Elucidating microstructural evolution and strengthening mechanisms in nanocrystalline surface induced by surface mechanical attrition treatment of stainless steel. Acta Mater. 2017, 122, 138–151. [Google Scholar] [CrossRef]
- Ba, D.M.; Ma, S.N. Progress in Researches on Mechanically Induced Surface Self-nanocrystallization Technology of Metallic Materials. Mater. Rep. 2006, 20, 92–95. [Google Scholar]
- Jentzsch, W.D.; Böhmer, S. Investigations on nitride layer formation at the iron surface during gas nitriding. Krist. Tech. 1979, 14, 617–624. [Google Scholar] [CrossRef]
- Tong, W.P.; He, C.S.; He, J.C.; Zuo, L.; Tao, N.; Wang, Z. Strongly enhanced nitriding kinetics by means of grain refinement. Appl. Phys. Lett. 2006, 89, 021918. [Google Scholar] [CrossRef]
- Sun, J.; Tong, W.P.; Zhang, H.; Du, X.D.; Wu, Y.C. Enhanced strength and plasticity of gas nitrided iron by surface mechanical attrition pretreatment. Surf. Coat. Technol. 2016, 286, 279–284. [Google Scholar] [CrossRef]
- Lu, L.; Sui, M.L.; Lu, K. Superplastic extensibility of nanocrystalline copper at room temperature. Science 2000, 287, 1463–1466. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Tao, N.R.; Wang, Z.B.; Tong, W.P.; Sui, M.L.; Lu, J.; Lu, K. An investigation of surface nanocrystallization mechanism in Fe induced by surface mechanical attrition treatment. Acta Mater. 2002, 50, 4603–4616. [Google Scholar] [CrossRef]
- Sun, J.; Tong, W.P.; Zhang, H.; Zuo, L.; Wang, Z.B. Evaluation of surface-modified 20CrMo by plasma nitriding coupled with ion sputtering and SMAT. Surf. Coat. Technol. 2012, 213, 247–252. [Google Scholar] [CrossRef]
- Tong, W.P.; Han, Z.; Wang, L.M.; Lu, J.; Lu, K. Low-temperature nitriding of 38CrMoAl steel with a nanostructured surface layer induced by surface mechanical attrition treatment. Surf. Coat. Technol. 2008, 202, 4957–4963. [Google Scholar] [CrossRef]
- Sun, J.; Tong, W.P.; Zuo, L.; Wang, Z.B. Low-temperature plasma nitriding of titanium layer on Ti/Al clad sheet. Mater. Des. 2013, 47, 408–415. [Google Scholar] [CrossRef]
- Chemkhi, M.; Retraint, D.; Roos, A.; Garnier, C.; Waltz, L.; Demangel, C.; Proust, G. The effect of surface mechanical attrition treatment on low temperature plasma nitriding of an austenitic stainless steel. Surf. Coat. Technol. 2013, 221, 191–195. [Google Scholar] [CrossRef]
- Balusamy, T.; Narayanan, T.S.N.S.; Ravichandran, K.; Park, I.S.; Lee, M.H. Plasma nitriding of AISI 304 stainless steel: Role of surface mechanical attrition treatment. Mater. Charact. 2013, 85, 38–47. [Google Scholar] [CrossRef]
- Lin, Y.M.; Lu, J.; Wang, L.P.; Xu, T.; Xue, Q.J. Surface nanocrystallization by surface mechanical attrition treatment and its effect on structure and properties of plasma nitrided AISI 321 stainless steel. Acta Mater. 2006, 54, 5599–5605. [Google Scholar] [CrossRef]
- Zhang, H.; Qin, H.F.; Ren, Z.C.; Zhao, J.Y.; Hou, X.N.; Doll, G.L.; Dong, Y.L.; Ye, C. Low-temperature nitriding of nanocrystalline Inconel 718 alloy. Surf. Coat. Technol. 2017, 330, 10–16. [Google Scholar] [CrossRef]
- Liu, D.; Liu, D.X.; Zhang, X.H.; Liu, C.S.; Zhao, W.D.; Xu, X.C. Research Progress in Effect of Surface Deformation Nano-crystalline on Nitriding Behavior of Metallic Materials. Rare Met. Mater. Eng. 2019, 48, 1352–1360. [Google Scholar]
- Sun, J.; Mei, L.Y.; Li, Y.; Lei, Y.Y.; Du, X.D.; Wu, Y.C. Two-Step Nitriding Behavior of Pure Iron with a Nanostructured Surface Layer. Adv. Eng. Mater. 2019, 21, 1900359. [Google Scholar] [CrossRef]
- Proust, G.; Retraint, D.; Chemkhi, M.; Roos, A.; Demangel, C. Electron backscatter diffraction and transmission Kikuchi diffraction analysis of an austenitic stainless steel subjected to surface mechanical attrition treatment and plasma nitriding. Microsc. Microanal. 2015, 21, 919–926. [Google Scholar] [CrossRef]
- Tong, W.P.; Sun, J.; Zuo, L.; He, J.C.; Lu, J. Study on wear and friction resistance of nanocrystalline Fe nitrided at low temperature. Wear 2011, 271, 653–657. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.; Ma, C. Ultrasonic Nano-Crystal Surface Modification Assisted Gas Nitriding of Ti6Al4V Alloy. In Proceedings of the ASME 2017 12th International Manufacturing Science and Engineering Conference collocated with the JSME/ASME 2017 6th International Conference on Materials and Processing, Los Angeles, CA, USA, 4–8 June 2017. [Google Scholar]
- Zhang, C.S.; Wang, Y.; Chen, D.Z.; Wu, Y.Q.; Cui, G.D.; Yang, Y.; Wang, Y.X.; Chen, Y.X. Effect of elemental doping on the catalytic activity of ABO3 perovskite oxides during low-pressure gas nitriding. Appl. Surf. Sci. 2021, 542, 148706. [Google Scholar] [CrossRef]
- Aleksandrov, V.A.; Ostaeva, G.Y.; Papisova, A.I.; Papisov, I.M.; Petrova, L.G.; Prikhodko, V.M.; Fatyukhin, D.S. Synthesis of copper–polymer nanocomposite on steel surface and composite-based catalyst for steel nitriding. Colloid J. 2015, 77, 556–560. [Google Scholar] [CrossRef]
- Kang, K.; Kwon, S.; Lee, C.; Hong, D.; Lee, H.M. Hierarchical analysis of alloying element effects on gas nitriding rate of Fe alloys: A DFT, microkinetic and kMC study. Acta Mater. 2019, 174, 173–180. [Google Scholar] [CrossRef]
- Nakai, M.; Niinomi, M.; Akahori, T.; Ohtsu, N.; Nishimura, H.; Toda, H.; Fukui, H.; Ogawa, M. Effects of alloying elements on hard ceramic layer formation on surfaces of biomedical Ti-29Nb-13Ta-4.6 Zr and Ti-6Al-4V ELI during gas nitriding. J. Jpn. Inst. Met. 2007, 71, 415–422. [Google Scholar] [CrossRef]
- Yang, M.; Sisson, R.D. Alloy effects on the gas nitriding process. J. Mater. Eng. Perform. 2014, 23, 4181–4186. [Google Scholar] [CrossRef]
- Bell, T.; Sun, Y.; Lui, Z.; Yan, M. Rare-earth surface engineering. Heat Treat. Met. 2000, 27, 1–8. [Google Scholar]
- Cleugh, D.; Blawert, C.; Steinbach, J.; Ferkel, H.; Mordike, B.L.; Bell, T. Effects of rare earth additions on nitriding of EN40B by plasma immersion ion implantation. Surf. Coat. Technol. 2001, 142, 392–396. [Google Scholar] [CrossRef]
- Dossett, J.; Totten, G.E. Fundamentals of nitriding and nitrocarburizing. In ASM Handbook: Steel Heat Treating Fundamentals and Processes; ASM International: Materials Park, OH, USA, 2013; p. 619. [Google Scholar]
- Podgurski, H.H.; Davis, F.N. Thermochemistry and nature of nitrogen absorption in nitrogenated Fe Ti alloys. Acta Metall. 1981, 29, 1–9. [Google Scholar] [CrossRef]
- Steiner, T.; Mittemeijer, E.J. Alloying element nitride development in ferritic Fe-based materials upon nitriding: A review. J. Mater. Eng. Perform. 2016, 25, 2091–2102. [Google Scholar] [CrossRef]
- Meka, S.R.; Jung, K.S.; Bischoff, E.; Mittemeijer, E.J. Unusual precipitation of amorphous silicon nitride upon nitriding Fe–2at.% Si alloy. Philos. Mag. 2012, 92, 1435–1455. [Google Scholar] [CrossRef]
- Somers, M.A.J.; Lankreijer, R.M.; Mittemeijer, E.J. Excess nitrogen in the ferrite matrix of nitrided binary iron-based alloys. Philos. Mag. A 1989, 59, 353–378. [Google Scholar] [CrossRef]
- Steiner, T.; Meka, S.R.; Bischoff, E.; Waldenmaier, T.; Mittemeijer, E.J. Nitriding of ternary Fe–Cr–Mo alloys; role of the Cr/Mo-ratio. Surf. Coat. Technol. 2016, 291, 21–33. [Google Scholar] [CrossRef]
- Steiner, T.; Meka, S.R.; Rheingans, B.; Bischoff, E.; Waldenmaier, T.; Yeli, G.; Martin, T.L.; Bagot, P.A.J.; Moody, M.P.; Mittemeijer, E.J. Continuous and discontinuous precipitation in Fe-1 at.% Cr-1 at.% Mo alloy upon nitriding; crystal structure and composition of ternary nitrides. Philos. Mag. 2016, 96, 1509–1537. [Google Scholar] [CrossRef]
- Inia, D.K.; Arnoldbik, W.M.; Vredenberg, A.M.; Boerma, D.O. New method for low temperature gaseous nitriding of iron. Surf. Eng. 1996, 12, 326–330. [Google Scholar] [CrossRef]
- Inia, D.K.; Pröpper, M.H.; Arnoldbik, W.M.; Vredenberg, A.M.; Boerma, D.O. Low-temperature nitriding of iron through a thin nickel layer. Appl. Phys. Lett. 1997, 70, 1245–1247. [Google Scholar] [CrossRef]
- Mao, C.J.; Wei, K.X.; Liu, X.L.; Yu, X.H.; Hu, J. A novel titanium enhanced plasma nitriding for 42CrMo steel. Mater. Lett. 2020, 262, 127052. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, K.; Chang, J.N.; Qu, S.W.; Jia, W.R.; Li, Z.B.; Yu, S.W.; Gao, J.; Ma, Y. Microstructure, mechanical properties and reciprocating wear properties of diamond grits-reinforced NiCrBSi composite coatings on 42CrMo. Surf. Coat. Technol. 2022, 445, 128703. [Google Scholar] [CrossRef]
- Wei, Y.D.; Liu, Z.R.; Wang, C.Y.; Fan, A.L.; Chen, J.M. A note on coating of surface diffusion-infiltration of Re on steel 20 and armco iron by chemical process. Acta Metall. Sin. 1983, 19, 121–124. [Google Scholar]
- Yan, M.F.; Liu, Z.R.; Zhu, F.Y. Progress in Rare Earth Thermochemical Treatment. Heat Treat. Met. 2003, 28, 1–6. [Google Scholar]
- Jiang, J.H.; Jiang, J.Q.; Ma, A.B.; Zhang, X.H. Application of Rare Earth Addition on the Chemical Heat-treatment. China Surf. Eng. 2003, 16, 10–14. [Google Scholar]
- Hao, S.; Wei, Y.D.; Huang, J.X.; Sun, L. Conducting Materials of PbTiO3 Ceramics Prepared by Gaseous Penetration of Rare Earth. Rare Met. Mater. Eng. 2005, 34, 1361–1364. [Google Scholar]
- Zhao, W.J.; Liu, G.Q.; Cai, H.; Liu, R.; Chen, C.; Guo, Y.; Wu, Y.P.; Yu, Q.F. Research on Rare Earth Carburized Process of 20Cr2Ni4A Gear Steel. Foundry 2018, 67, 831–835. [Google Scholar]
- Li, S.X.; Gu, M.; Sun, Q.F. Energized function of Ce-rich rare earth content on ion nitriding. Heat Treat. Met. 2019, 44, 93–96. [Google Scholar]
- Pu, S.J.; Yang, H.P.; Wang, H.B.; Wu, X.C. Effect of Rare Earth Ce on High Temperature Friction and Wear Property of Pack Boronized H13 Steel. Chin. J. Mater. Res. 2015, 29, 481–488. [Google Scholar]
- Adijanto, L.; Padmanabhan, V.B.; Holmes, K.J.; Gorte, R.J.; Vohs, J.M. Physical and electrochemical properties of alkaline earth doped, rare earth vanadates. J. Solid State Chem. 2012, 190, 12–17. [Google Scholar] [CrossRef]
- Cai, X.Y.; Chen, Q.W.; Dai, P.Q.; Yan, Q. Study on Rare Earth Carbonitriding Technology of 20CrMnTi Steel. Hot Work. Technol. 2018, 47, 233–235. [Google Scholar]
- Zhang, G.L.; Xiang, W.M.; Liu, Z.R.; Liu, C.Y. The Rare Earth Boost Infiltrating Technology and the Process. Heat Treat. Technol. Equip. 2009, 30, 15–21. [Google Scholar]
- Wang, B.X.; Zhang, G.L.; Liu, C.Y. Brief Analysis of Rare Earth Nitriding Mechanism. Heat Treat. Technol. Equip. 2013, 34, 64–69. [Google Scholar]
- Li, Q.F.; Li, L.; Liu, E.B.; Liu, D.; Cui, X.F. Temper embrittlement dynamics induced by non-equilibrium segregation of phosphorus in steel 12Cr1MoV. Scr. Mater. 2005, 53, 309–313. [Google Scholar] [CrossRef]
- Torchane, L. Influence of rare earths on the gas nitriding kinetics of 32CrMoNiV5 steel at low temperature. Surf. Interfaces 2021, 23, 101016. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Arabczyk, W. Studies of the Ammonia Decomposition over a Mixture Of α-Fe (N) And γ′-Fe4n. Pol. J. Chem. Technol. 2013, 15, 97–101. [Google Scholar]
- Yasuda, N.; Mochizuki, Y.; Tsubouchi, N.; Akiyama, T. Reduction and nitriding behavior of hematite with ammonia. ISIJ Int. 2015, 55, 736–741. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Sankaran, A.; Kamruddin, M.; Nithya, R.; Shankar, P.; Dash, S.; Tyagi, A.K.; Raj, B. Morphology and growth aspects of Cr (N) phases on gas nitridation of electroplated chromium on AISI 316 LN stainless steel. Surf. Coat. Technol. 2006, 201, 102–107. [Google Scholar] [CrossRef]
- Vitry, V.; Kanta, A.F.; Delaunois, F. Application of nitriding to electroless nickel–boron coatings: Chemical and structural effects; mechanical characterization; corrosion resistance. Mater. Des. 2012, 39, 269–278. [Google Scholar] [CrossRef]
- Peng, T.T.; Dai, M.Y.; Cai, W.; Wei, W.; Wei, K.X.; Hu, J. The enhancement effect of salt bath preoxidation on salt bath nitriding for AISI 1045 steel. Appl. Surf. Sci. 2019, 484, 610–615. [Google Scholar] [CrossRef]
- Li, J.C.; Sun, F.; Wang, S.K.; Yang, X.M.; Hu, J. Catalysis effect and mechanism of pre-oxidation on direct current plasma nitriding. Trans. Mater. Heat Treat. 2014, 7, 182–186. [Google Scholar]
- Zhen, J.Z.; Shi, Q.W.; Shi, J.; Liu, J.X. Research Progress of Low Temperature Surface Nitriding Technology for Steel Materials. Hot Work. Technol. 2019, 48, 35–40. [Google Scholar]
- Liu, H.; Li, J.C.; Sun, F.; Hu, J. Characterization and effect of pre-oxidation on DC plasma nitriding for AISI4140 steel. Vacuum 2014, 109, 170–174. [Google Scholar] [CrossRef]
- Güntherschulze, A.; Betz, H. Neue Untersuchungen über die elektrolytische Ventilwirkung: V. Die Eigenschaften der Funken. Z. Phys. 1932, 78, 196–210. [Google Scholar] [CrossRef]
- Snezhko, L. Anodic Process in Forming of Silicate Coatings.(Translation). Prot. Met. 1984, 20, 243–245. [Google Scholar]
- Zhang, J.T.; Liu, Z.H.; Sun, J.X.; Zhao, H.L.; Shi, Q.Y.; Ma, D.W. Microstructure and mechanical property of electropulsing tempered ultrafine grained 42CrMo steel. Mater. Sci. Eng. 2020, 782, 139213. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Ma, N. Catalysed gas nitriding process for 30Cr3MoA steel. Heat Treat. Met. 2019, 44, 150–153. [Google Scholar]
- Liu, J.; Liu, Y.J.; Lu, X.F. Investigation on Corrosion Performance of Vacuum Nitriding Layer on 25Cr3Mo. Foundry Technol. 2013, 34, 695–697. [Google Scholar]
- Wang, Z.L. Effect of pre-oxidation on microstructure and properties of the nitrided layer of 40Cr steel prepared by seal pot method. Heat Treat. Met. 2019, 44, 119–123. [Google Scholar]
- Li, J.C.; Yang, X.M.; Wang, S.K.; Wei, K.X.; Hu, J. A rapid DC plasma nitriding technology catalyzed by pre-oxidation for AISI4140 steel. Mater. Lett. 2014, 116, 199–202. [Google Scholar] [CrossRef]
- Tang, L.; Mao, C.J.; Jia, W.J.; Wei, K.X.; Hu, J. The effect of novel composite pretreatment on performances of plasma nitrided layer. J. Mater. Res. Technol. 2020, 9, 9531–9536. [Google Scholar] [CrossRef]
- Hur, I.C.; Son, K.S.; Yoon, J.H.; Cho, T.Y.; Park, B.G.; Kim, H.S.; Kim, I.S. A study on surface properties and high temperature oxidation behavior of ion nitrided FC-25 gray cast iron. J. Korean Inst. Met. Mater. 2005, 43, 298–305. [Google Scholar]
- Poncet, M.; Issartel, C.; Perrier, S.; Buscail, H. Atmosphere influence on oxidation at high temperature of ni–cr–si model alloys. Oxid. Met. 2021, 96, 117–127. [Google Scholar] [CrossRef]
- Tang, L.; Zhao, X.B.; Li, J.C.; Wei, K.X.; Hu, J. Effect of Pre-Oxidation on Plasma Nitrided Steel AISI4140. Met. Sci. Heat Treat. 2020, 62, 224–228. [Google Scholar] [CrossRef]
- Boynazarov, U.R.; Petrova, L.G.; Brezhnev, A.A.; Bibikov, P.S. Properties of Oxynitride Steel Coatings Obtained Through Three-Stage Processes of Nitriding Combined with Oxidation. Metallurgist 2021, 65, 886–892. [Google Scholar] [CrossRef]
- Lee, S.Y. Mechanical properties of TiNx/Cr1−xN thin films on plasma nitriding-assisted AISI H13 steel. Surf. Coat. Technol. 2005, 193, 55–59. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Sahin, A.Z.; Ayar, T.; Aleem, B.J.A. Laser nitriding of steel surface: Efficiency analysis. Int. J. Surf. Sci. Eng. 2010, 4, 492–504. [Google Scholar] [CrossRef]
- Yan, G.H.; Lu, S.L.; Zhang, M.L.; Wang, J.C.; Yang, X.D.; Zhang, Z.Y.; Gu, J.F.; Li, C.W. Wear and corrosion behavior of P20 steel surface modified by gas nitriding with laser surface engineering. Appl. Surf. Sci. 2020, 530, 147306. [Google Scholar] [CrossRef]
- Hwang, T.W.; Han, S.W.; Oh, I.Y.; Moon, Y.H. Characterization of the Laser Surface Nitriding of Ti-6Al-4V Alloys in Nitric Acid Solution and Nitrogen Gas Atmosphere. Korean J. Met. Mater. 2019, 57, 412–421. [Google Scholar] [CrossRef]
- Wang, B.; Liu, B.; Zhang, X.D.; Gu, J.F. Enhancing heavy load wear resistance of AISI 4140 steel through the formation of a severely deformed compound-free nitrided surface layer. Surf. Coat. Technol. 2018, 356, 89–95. [Google Scholar] [CrossRef]
- Tinubu, O.O.; Das, S.; Dutt, A.; Mogonye, J.E.; Ageh, V.; Xu, R.; Forsdike, J.; Mishra, R.S.; Scharf, T.W. Friction stir processing of A-286 stainless steel: Microstructural evolution during wear. Wear 2016, 356, 94–100. [Google Scholar] [CrossRef]
- Zong, X.; Wang, H.M.; Li, J.; Cheng, X.; Li, Z.; Tang, H.B. Microstructure characterization and evolution mechanism of titanium during laser surface nitriding. Mater. Charact. 2022, 190, 112029. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, K.; Chen, C.J.; Li, Y.; Wang, X.N.; Hu, Z.R.; Zhang, S.H. Study on TC4 Titanium Alloy used as Valve by Laser Surface Nitriding. Appl. Laser 2015, 35, 408–414. [Google Scholar]
- Wang, C.; Hong, J.; Cui, M.M.; Huang, H.; Zhang, L.; Yan, J.W. The effects of simultaneous laser nitriding and texturing on surface hardness and tribological properties of Ti6Al4V. Surf. Coat. Technol. 2022, 437, 128358. [Google Scholar] [CrossRef]
- Zhecheva, A.; Sha, W.; Malinov, S.; Long, A. Enhancing the microstructure and properties of titanium alloys through nitriding and other surface engineering methods. Surf. Coat. Technol. 2005, 200, 2192–2207. [Google Scholar] [CrossRef]
- Pérez Artieda, M.G.; Fernández Carrasquilla, J. Revisión sobre nitruraciones láser de aleaciones de titanio. Rev. De Metal. 2010, 46, 530–541. [Google Scholar] [CrossRef]
- Zhao, N.Q.; Man, H.C.; Cui, Z.D.; Yang, X.J. Structure and wear properties of laser gas nitrided NiTi surface. Surf. Coat. Technol. 2006, 200, 4879–4884. [Google Scholar] [CrossRef]
- Feng, Z.H.; Sun, X.Y.; Han, P.B.; Fu, H.; Dong, H.C.; Guo, S.; Su, R.; Li, J.H. Microstructure and microhardness of a novel TiZrAlV alloy by laser gas nitriding at different laser powers. Rare Met. 2020, 39, 270–278. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Manikandan, M.; Shukla, A.K.; Subramaniam, S.; Balaji, V.P.; Palani, I.A.; Jayaprakash, M. Influence of laser-nitriding on mechanical and elevated temperature fretting wear behavior of A356-alloy. Surf. Coat. Technol. 2021, 413, 127072. [Google Scholar] [CrossRef]
- Adachi, S.; Yamaguchi, T.; Ueda, N. Formation and Properties of Nitrocarburizing S-Phase on AISI 316L Stainless Steel-Based WC Composite Layers by Low-Temperature Plasma Nitriding. Metals 2021, 11, 1538. [Google Scholar] [CrossRef]
- Kowalska, J.; Małdziński, L. ZeroFlow-new, environmentally friendly method of controlled gas nitriding used for selected car parts. In IOP Conference Series: Materials Science and Engineering, Proceedings of the Scientific Conference on Automotive Vehicles and Combustion Engines (KONMOT 2016), Krakow, Poland, 22–23 September 2016; IOP Publishing: Bristol, UK, 2016; Volume 148, p. 012047. [Google Scholar]
- Triki, A.; Gravey, A.; Gravey, P. CAPEX and OPEX saving in SDN-compliant sub-wavelength switching solution. In Proceedings of the 2015 International Conference on Photonics in Switching (PS), Florence, Italy, 22–25 September 2015; pp. 262–264. [Google Scholar]
- Feng, R.; Lin, P.N.; Hou, C.X.; Jia, S.S. Study of the Effect of China’s Emissions Trading Scheme on Promoting Regional Industrial Carbon Emission Reduction. Front. Resour. Effic. Environ. Impact Assess. 2023, 16648714, 51. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Tong, W.P.; Gong, M.Y.; Li, H.Z.; Chen, L.Q. Nitriding of nanocrystalline pure Fe induced by surface mechanical attrition treatment[C]//MATEC Web of Conferences. EDP Sci. 2015, 21, 08009. [Google Scholar]
- Weschenfelder, S.E.; Mello, A.C.C.; Borges, C.P.; Campos, J.C. Oilfield produced water treatment by ceramic membranes: Preliminary process cost estimation. Desalination 2015, 360, 81–86. [Google Scholar] [CrossRef]
- Matera, F.; Listanti, M.; Pióro, M. Recent trends in network planning to decrease the CAPEX/OPEX cost. Telecommun. Syst. 2016, 61, 205–207. [Google Scholar] [CrossRef]
- Baron, P.; Cornet, S.M.; Collins, E.D.; DeAngelis, G.; Cul, G.D.; Fedorov, Y.; Glatz, J.P.; Ignatiev, V.; Inoue, T.; Khaperskaya, A.; et al. A review of separation processes proposed for advanced fuel cycles based on technology readiness level assessments. Prog. Nucl. Energy 2019, 117, 103091. [Google Scholar] [CrossRef]
- Straub, J. In search of technology readiness level (TRL) 10. Aerosp. Sci. Technol. 2015, 46, 312–320. [Google Scholar] [CrossRef]
- Cozzi, L.; Gould, T.; Bouckart, S. World Energy Outlook 2020; IEA: Paris, France, 2020; Volume 2050, pp. 1–461. [Google Scholar]
- Neumann, J.; Da Rocha, R.C.; Debiagi, P.; Scholtissek, A.; Dammel, F.; Stephan, P.; Hasse, C. Techno-economic assessment of long-distance supply chains of energy carriers: Comparing hydrogen and iron for carbon-free electricity generation. Appl. Energy Combust. Sci. 2023, 14, 100128. [Google Scholar] [CrossRef]
- Devlin, A.; Kossen, J.; Goldie-Jones, H.; Yang, A.D. Global green hydrogen-based steel opportunities surrounding high quality renewable energy and iron ore deposits. Nat. Commun. 2023, 14, 2578. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, H.; Zhang, F.J. The Technology Readiness Analysis of Electronic Information System. In Proceedings of the 4th International Conference on Computer, Mechatronics, Control and Electronic Engineering, Hangzhou, China, 28–29 September 2015; Atlantis Press: Dordrecht, The Netherlands, 2015; pp. 845–849. [Google Scholar]
- Yaskiv, O.I.; Pohrelyuk, I.M.; Fedirko, V.M.; Lee, D.B.; Tkachuk, O.V. Formation of oxynitrides on titanium alloys by gas diffusion treatment. Thin. Solid Film. 2011, 519, 6508–6514. [Google Scholar] [CrossRef]
- Yoshida, M.; Ichiki, R.; Utsumi, N. Surface hardening of titanium using gas nitriding. Int. J. Precis. Eng. Manuf. 2013, 14, 971–976. [Google Scholar] [CrossRef]
- Siyahjani, F.; Atar, E.; Cimenoglu, H. Structural changes on the surface of alloy Ti6Al7Nb under gas nitriding. Met. Sci. Heat Treat. 2016, 58, 170–174. [Google Scholar] [CrossRef]
- Baranowska, J.; Szczeciński, K.; Wysiecki, M. Increasing of gas nitriding kinetics via surface pre-treatment. Surf. Coat. Technol. 2002, 151, 534–539. [Google Scholar] [CrossRef]
- Lacaille, V.; Peres, V.; Langlade, C.; Morel, C.; Feulvarch, E.; Bergheau, J.M.; Kermouche, G. Combination of mechanical and chemical pre-treatments to improve nitriding efficiency on pure iron. Appl. Surf. Sci. 2017, 414, 73–81. [Google Scholar] [CrossRef]
- Díaz-Guillén, J.C.; Naeem, M.; Hdz-García, H.M.; Acevedo-Davila, J.L.; Diaz-Guillen, M.R.; Khan, M.A.; Iqbal, J.; Mtz-Enriquez, A.T. Duplex plasma treatment of AISI D2 tool steel by combining plasma nitriding (with and without white layer) and post-oxidation. Surf. Coat. Technol. 2020, 385, 125420. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.Y.; Wu, G.L.; Li, L.; Yao, J.H.; Zhang, Q.L. The microstructure and cavitation erosion resistance of Ti6Al4V alloy treated by laser gas nitriding with scanning galvanometer. Opt. Laser Technol. 2022, 153, 108270. [Google Scholar] [CrossRef]



















| Process | Penetrant | Activator | Filler | Rare Earth |
|---|---|---|---|---|
| Monopermeable rare earth [152] | - | NH4Cl, KCl | Al2O3 | Mixed ReCl, rare-earth metal nuggets (powder) |
| Rare-earth carburizing (Gas) [153] | Kerosene or natural gas | - | Methanol | ReCl, ReO, rare-earth powder |
| Rare-earth nitriding (Gas) [154] | Ammonia | - | Methanol | ReCl |
| Rare-earth boronizing [155] | B4C, ferroboron | Al | SiC | ReCl, ReO, rare-earth powder |
| Rare-earth vanadium [156] | V2O5 | Al, Si | SiC | Misch metal |
| Rare-earth carbonitriding [157] | Carburization | Fluoride | Methanol | ReCl |
| TRL | Function | Definition |
|---|---|---|
| 1–2 | Proof of concept | Basic research is carried out in the laboratory, and further experimental verification and improvement are still needed. |
| 3–4 | - | Technical verification and prototype development are carried out in the laboratory, and further engineering verification is still required. |
| 5–6 | Proof of principle | Engineering verification and field demonstrations in laboratories and factories have reached a certain level of maturity, but further expansion and improvement are still needed. |
| 7–8 | - | Large-scale application and verification in the actual production environment has achieved practical application results. |
| 9 | Proof of performance | It has been widely used and achieved commercial success. |
| Process | Technology Readiness Level | Reference Project Status and R&D | CO2 Emission Reduction Potential | Commercial Application | Ref. |
|---|---|---|---|---|---|
| Conventional gas nitriding | 8 | - | 5 | 8 | [192,210] |
| Process parameter optimization | 7 | - | 3 | 7 | [211,212] |
| Surface mechanical nano-crystallization | 6 | Researchers at the KTH Royal Institute of Technology in Stockholm, Sweden, are investigating the use of nano-processing to improve the nitriding process. | 6 | 5 | [213,214] |
| Surface-active catalytic nitriding | 5 | Researchers at the University of Duisburg-Essen in Germany are studying the use of transition metals, such as molybdenum, tungsten, and chromium, as catalysts to promote nitriding reactions. | 7 | 4 | [131,213] |
| Surface pre-oxidized nitriding | 5 | Researchers at the University of Surrey in the UK are investigating the use of oxidation pretreatments to improve the nitriding process. | 4 | 3 | [181,215] |
| Surface laser treatment | 3 | Researchers at the Swiss Federal Institute of Technology in Zurich are investigating the use of laser processing to improve the nitriding process. | 8 | 2 | [103,216] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-L.; Xia, F.; Xie, A.-J.; Peng, H.-P.; Wang, J.-H.; Li, Z.-W. A Review—Effect of Accelerating Methods on Gas Nitriding: Accelerating Mechanism, Nitriding Behavior, and Techno-Economic Analysis. Coatings 2023, 13, 1846. https://doi.org/10.3390/coatings13111846
Zhou Y-L, Xia F, Xie A-J, Peng H-P, Wang J-H, Li Z-W. A Review—Effect of Accelerating Methods on Gas Nitriding: Accelerating Mechanism, Nitriding Behavior, and Techno-Economic Analysis. Coatings. 2023; 13(11):1846. https://doi.org/10.3390/coatings13111846
Chicago/Turabian StyleZhou, Yu-Long, Fan Xia, Ai-Jun Xie, Hao-Ping Peng, Jian-Hua Wang, and Zhi-Wei Li. 2023. "A Review—Effect of Accelerating Methods on Gas Nitriding: Accelerating Mechanism, Nitriding Behavior, and Techno-Economic Analysis" Coatings 13, no. 11: 1846. https://doi.org/10.3390/coatings13111846
APA StyleZhou, Y. -L., Xia, F., Xie, A. -J., Peng, H. -P., Wang, J. -H., & Li, Z. -W. (2023). A Review—Effect of Accelerating Methods on Gas Nitriding: Accelerating Mechanism, Nitriding Behavior, and Techno-Economic Analysis. Coatings, 13(11), 1846. https://doi.org/10.3390/coatings13111846