Effect of One Sulfate-Reducing Bacterium SRB-Z Isolated from Pearl River on the Corrosion Behavior of Q235 Carbon Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Screening, and Purification of Bacteria Strains
2.2. Metal Material and Immersion Corrosion Test
2.3. Morphology of Biofilm and Pit Measurement
2.4. Electrochemical Corrosion Analysis
2.5. H2S Measurement
3. Results
3.1. Morphology and Composition of Biofilm and Corrosion Product
3.2. Pit Morphology and Pit Depth
3.3. Electrochemical Measurements
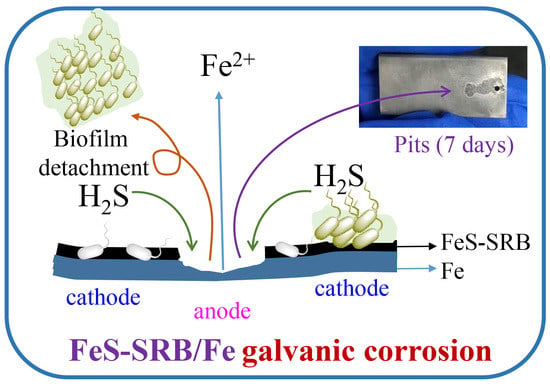
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lou, Y.; Chang, W.; Cui, T.; Wang, J.; Qian, H.; Ma, L.; Hao, X.; Zhang, D. Microbiologically influenced corrosion inhibition mechanisms in corrosion protection: A review. Bioelectrochemistry 2021, 141, 107883. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeter. Biodegr. 2019, 137, 42–58. [Google Scholar] [CrossRef]
- Zhou, E.; Li, F.; Zhang, D.; Xu, D.; Li, Z.; Jia, R.; Jin, Y.; Song, H.; Li, H.; Wang, Q.; et al. Direct microbial electron uptake as a mechanism for stainless steel corrosion in aerobic environments. Water Res. 2022, 219, 118553. [Google Scholar] [CrossRef] [PubMed]
- Little, B.J.; Blackwood, D.J.; Hinks, J.; Lauro, F.M.; Marsili, E.; Okamoto, A.; Rice, S.A.; Wade, S.A.; Flemming, H.C. Microbially influenced corrosion—Any progress? Corros. Sci. 2020, 170, 108641. [Google Scholar] [CrossRef]
- Vigneron, A.; Head, I.M.; Tsesmetzis, N. Damage to offshore production facilities by corrosive microbial biofilms. Appl. Microb. Biotech. 2018, 102, 2525–2533. [Google Scholar] [CrossRef]
- Vigneron, A.; Alsop, E.B.; Chambers, B.; Lomans, B.P.; Head, I.M.; Tsesmetzis, N. Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Appl. Environ. Microb. 2016, 82, 2545–2554. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Yan, M.; Yu, L.; Zhao, H.; Sun, C.; Yin, F.; Ke, W. Stress corrosion of pipeline steel under disbonded coating in a SRB-containing environment. Corros. Sci. 2019, 157, 518–530. [Google Scholar] [CrossRef]
- Wu, T.; Sun, C.; Ke, W. Interpreting microbiologically assisted cracking with E(e)-pH diagrams. Bioelectrochemistry 2018, 120, 57–65. [Google Scholar] [CrossRef]
- Wu, T.; Yan, M.; Zeng, D.; Xu, J.; Sun, C.; Yu, C.; Ke, W. Stress corrosion cracking of X80 steel in the presence of sulfate-reducing bacteria. J. Mater. Sci. Technol. 2015, 31, 413–422. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Yan, M.; Sun, C.; Yu, C.; Ke, W. Synergistic effect of sulfate-reducing bacteria and elastic stress on corrosion of X80 steel in soil solution. Corros. Sci. 2014, 83, 38–47. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, J.; Li, G.; Liu, H. Crevice corrosion of X80 carbon steel induced by sulfate reducing bacteria in simulated seawater. Bioelectrochemistry 2021, 142, 107933. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Liu, J.; Cai, W.; Wang, D.; Jia, R.; Chen, S.; Gu, T. Electrochemical investigation of increased carbon steel corrosion via extracellular electron transfer by a sulfate reducing bacterium under carbon source starvation. Corros. Sci. 2019, 150, 258–267. [Google Scholar] [CrossRef]
- Li, Y.; Feng, S.; Liu, H.; Tian, X.; Xia, Y.; Li, M.; Xu, K.; Yu, H.; Liu, Q.; Chen, C. Bacterial distribution in SRB biofilm affects MIC pitting of carbon steel studied using FIB-SEM. Corros. Sci. 2020, 167, 108512. [Google Scholar] [CrossRef]
- Wang, D.; Kijkla, P.; Mohamed, M.E.; Saleh, M.A.; Kumseranee, S.; Punpruk, S.; Gu, T. Aggressive corrosion of carbon steel by Desulfovibrio ferrophilus IS5 biofilm was further accelerated by riboflavin. Bioelectrochemistry 2021, 142, 107920. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, C.; Wang, P.; Zhang, D. Fabrication of a robust slippery liquid infused porous surface on Q235 carbon steel for inhibiting microbiologically influenced corrosion. Colloid. Surface A Physicochem. Eng. Asp. 2021, 631, 127696. [Google Scholar] [CrossRef]
- Guan, F.; Duan, J.; Zhai, X.; Wang, N.; Zhang, J.; Lu, D.; Hou, B. Interaction between sulfate-reducing bacteria and aluminum alloys—Corrosion mechanisms of 5052 and Al-Zn-In-Cd aluminum alloys. J. Mater. Sci. Technol. 2020, 36, 55–64. [Google Scholar] [CrossRef]
- Gu, T.; Wang, D.; Lekbach, Y.; Xu, D. Extracellular electron transfer in microbial biocorrosion. Curr. Opin. Electrochem. 2021, 29, 100763. [Google Scholar] [CrossRef]
- Wei, B.; Xu, J.; Cheng, Y.F.; Sun, C.; Yu, C.; Wang, Z. Effect of uniaxial elastic stress on corrosion of X80 pipeline steel in an acidic soil solution containing sulfate-reducing bacteria trapped under disbonded coating. Corros. Sci. 2021, 193, 109893. [Google Scholar] [CrossRef]
- Davidova, I.A.; Lenhart, T.R.; Nanny, M.A.; Suflita, J.M. Composition and corrosivity of extracellular polymeric substances from the hydrocarbon-degrading sulfate-reducing bacterium Desulfoglaeba alkaexedens ALDC. Microorganisms 2021, 9, 1994. [Google Scholar] [CrossRef]
- Wang, D.; Yang, C.; Saleh, M.A.; Alotaibi, M.D.; Mohamed, M.E. Conductive magnetite nanoparticles considerably accelerated carbon steel corrosion by electroactive Desulfovibrio vulgaris biofilm. Corros. Sci. 2022, 205, 110440. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, J.; Wang, Z.; Zhang, R.; Sand, W.; Zhang, L.; Duan, J.; Zhu, Q.; Hou, B. Corrosion of an AZ31B magnesium alloy by sulfate-reducing prokaryotes in a mudflat environment. Microorganisms 2022, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Salgar-Chaparro, S.J.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L.L. Microbiologically influenced corrosion as a function of environmental conditions: A laboratory study using oilfield multispecies biofilms. Corros. Sci. 2020, 169, 108595. [Google Scholar] [CrossRef]
- Sharma, M.; Liu, H.; Tsesmetzis, N.; Handy, J.; Place, T.; Gieg, L.M. Diagnosing microbiologically influenced corrosion at a crude oil pipeline facility leak site—A multiple lines of evidence approach. Int. Biodeter. Biodegr. 2022, 172, 105438. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Ru, J.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Venzlaff, H.; Enning, D.; Srinivasan, J.; Mayrhofer, K.J.J.; Hassel, A.W.; Widdel, F.; Stratmann, M. Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 2013, 66, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Biofilms, I.B.; Sztyler, M.; Gaylarde, C.C.; Smith, W.L.; Sunner, J. Biofilms and Biocorrosion; Woodhead Publishing Limited: Cambridge, UK, 2014. [Google Scholar]
- Lappin-Scott, H.; Burton, S.; Stoodley, P. Revealing a world of biofilms—the pioneering research of Bill Costerton. Nat. Rev. Microbiol. 2014, 12, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Ruan, H.M.; Zhang, G.A. Heterogeneous corrosion of mild steel under SRB-biofilm characterised by electrochemical mapping technique. Corr. Sci. 2011, 53, 2978–2987. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Microbial corrosion of X52 pipeline steel under soil with varied thicknesses soaked with a simulated soil solution containing sulfate-reducing bacteria and the associated galvanic coupling effect. Electrochim. Acta 2018, 266, 312–325. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Zhang, L.; Yang, J.; Lu, M.; Ding, J.; Li, H. Polymorphous FeS corrosion products of pipeline steel under highly sour conditions. Corros. Sci. 2016, 102, 103–113. [Google Scholar] [CrossRef]
- Dong, Y.; Song, G.L.; Zheng, D. Naturally effective inhibition of microbial corrosion by bacterium-alga symbiosis on 304 stainless steel. J. Clean. Prod. 2022, 356, 131823. [Google Scholar] [CrossRef]
- Wei, H.; Yang, X.Y.; van der Mei, H.C.; Busscher, H.J. X-ray photoelectron spectroscopy on microbial cell surfaces: A forgotten method for the characterization of microorganisms encapsulated with surface-engineered shells. Front. Chem. 2021, 9, 666159. [Google Scholar] [CrossRef] [PubMed]
- Kjaervik, M.; Ramstedt, M.; Schwibbert, K.; Dietrich, P.M.; Unger, W.E.S. Comparative study of NAP-XPS and Cryo-XPS for the investigation of surface chemistry of the bacterial cell-envelope. Front. Chem. 2021, 9, 666161. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D. Handbook of X-ray Photoelectron Spectroscopy, 3rd ed.; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979; p. 81. [Google Scholar]
- Shah, M.; Ayob, M.T.M.; Rosdan, R.; Yaakob, N.; Embong, Z.; Othman, N.K. The effect of H2S pressure on the formation of multiple corrosion products on 316L stainless steel surface. Sci. World J. 2020, 2020, 3989563. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fujiwara, Y. Effect of SO42− on the corrosion behavior of cerium-based conversion coatings on galvanized steel. Electrochim. Acta. 2006, 51, 4236–4242. [Google Scholar] [CrossRef]
- Katan, T.; Kargl, R.; Mohan, T.; Steindorfer, T.; Mozetič, M.; Kovač, J.; Kleinschek, K.S. Solid phase peptide synthesis on chitosan thin films. Biomacromolecules 2022, 23, 731–742. [Google Scholar] [CrossRef]
- Cha, B.J.; Choi, J.Y.; Ji, Y.; Zhao, S.; Kima, S.Y.; Kima, S.H.; Kima, Y.D. Fe-oxide/Al2O3 for the enhanced activity of H2S decomposition under realistic conditions: Mechanistic studies by in-situ DRIFTS and XPS. Chem. Eng. J. 2022, 443, 136459. [Google Scholar] [CrossRef]
- Liao, K.; Leng, J.; Cheng, Y.F.; He, T.; He, G.; Zhao, S.; Liu, X.; Huang, Q. Effect of H2S concentrations on corrosion failure of L245NS steel in CO2-O2-H2S system. Process. Saf. Environ. 2022, 168, 224–238. [Google Scholar] [CrossRef]
- Xu, H.; Wang, T.; Koppala, S.; Hu, J.; Ma, S.; Miao, W.; Le, T.; Zhang, L. Improving the quality of ammonium sulfate produced from the flue gas desulfurization process by using ammonium persulfate. Sep. Purif. Technol. 2023, 308, 122879. [Google Scholar] [CrossRef]
- Duret-Thual, C. Understanding corrosion: Basic principles. In Understanding Biocorrosion, 1st ed.; Liengen, T., Beech, I.B., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2014; Volume 1, pp. 3–32. [Google Scholar]
- Yuan, S.J.; Pehkonen, S.O.; Ting, Y.P.; Kang, E.T.; Neoh, K.G. Corrosion behavior of type 304 stainless steel in a simulated seawater-based medium in the presence and absence of aerobic Pseudomonas NCIMB 2021 bacteria. Ind. Eng. Chem. Res. 2008, 47, 3008–3020. [Google Scholar] [CrossRef]
- Huang, G.; Chan, K.Y.; Fang, H.H.P. Microbiologically induced corrosion of 70Cu-30Ni alloy in anaerobic seawater. J. Electrochem. Soc. 2004, 151, B434. [Google Scholar] [CrossRef] [Green Version]
- Ru, J.; Yang, D.; Xu, J.; Xu, D.; Gu, T. Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci. 2017, 127, 1–9. [Google Scholar]
- Cetin, D.; Aksu, M.L. Corrosion behavior of low-alloy steel in the presence of Desulfotomaculum sp. Corros. Sci. 2009, 51, 1584–1588. [Google Scholar] [CrossRef]
- Belkaid, S.; Ladjouzi, M.A.; Hamdani, S. Effect of biofilm on naval steel corrosion in natural seawater. J. Solid State Electrochem. 2011, 15, 525–537. [Google Scholar] [CrossRef]
- Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 2015, 100, 484–495. [Google Scholar]
- Nicoletti, D.; Sharma, M.; Gieg, L.M. Assessing microbial corrosion risk on offshore crude oil production topsides under conditions of nitrate and nitrite treatment for souring. Microorganisms 2022, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Zhao, H.; Zheng, S.; Chen, C. Initiation and developmental stages of steel corrosion in wet H2S environments. Corros. Sci. 2015, 93, 109–119. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, X.; Li, G.; Chen, S.; Quan, Z.; Zhao, S.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corr. Sci. 2000, 42, 1669–1683. [Google Scholar] [CrossRef]
- Tang, J.; Shao, Y.; Guo, J.; Zhang, T.; Meng, G.; Wang, F. The effect of H2S concentration on the corrosion behavior of carbon steel at 90 °C. Corr. Sci. 2010, 52, 2050–2058. [Google Scholar] [CrossRef]
- Beese-Vasbender, P.F.; Nayak, S.; Erbe, A.; Stratmann, M.; Mayrhofer, K.J.J. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim. Acta. 2015, 167, 321–329. [Google Scholar] [CrossRef]
- Aktas, D.F.; Sorrell, K.R.; Duncan, K.E.; Wawrik, B.; Callaghan, A.V.; Suflita, J.M. Anaerobic hydrocarbon biodegradation and biocorrosion of carbon steel in marine environments: The impact of different ultralow sulfur diesels and bioaugmentation. Int. Biodeterior. Biodegrad. 2017, 118, 45–56. [Google Scholar] [CrossRef] [Green Version]







| Composition 1 | Composition 3 | Composition 2 | |||
|---|---|---|---|---|---|
| MgSO4 | 2.0 g | Sodium Lactate | 3.5 g | K2HPO4 | 0.5 g |
| Sodium Citrate | 5.0 g | Yeast Extract | 1.0 g | DI water | 200.0 mL |
| CaSO4·H2O | 1.0 g | DI water | 200.0 mL | - | - |
| NH4Cl | 1.0 g | - | - | - | - |
| DI water | 400.0 mL | - | - | - | - |
| L-cysteine hydrochloride | 0.1 g | - | - | - | - |
| resazurin | 200 μL | - | - | - | - |
| Parameters | Conditions |
|---|---|
| Material | Q235 carbon steel |
| SRB strain | Desulfovibrio sp. (SRB-Z) |
| Culture medium | ATCC medium 1249 |
| Temperature | 37 °C |
| pH | 7.2 |
| Corrosion period | 7 days |
| Condition | βa (V dec−1) | βc (V dec−1) | Ecorr (V vs. Ag/AgCl) | Icorr (A cm−2) |
|---|---|---|---|---|
| none-SRB-Z | 0.122 | −0.109 | −0.721 | 1.03 × 10−6 |
| SRB-Z | 0.426 | −0.077 | −0.840 | 1.44 × 10−6 |
| Condition | t (d) | Qf (kΩ−1 sn cm−2) | Rf (Ω cm2) | Qdl (kΩ−1 sn cm−2) | Rct (kΩ cm2) |
|---|---|---|---|---|---|
| Sterile | 1 | 95.60 | 21.90 | 32.80 | 1.89 |
| - | 2 | 7.98 | 7.63 | 259.00 | 6.71 |
| - | 4 | 4.77 | 5.18 | 239.13 | 13.86 |
| - | 7 | 1.67 | 3.74 | 249.77 | 14.19 |
| - | 14 | 0.59 | 10.32 | 284.81 | 19.16 |
| SRB-Z-inoculated | 1 | 1.91 | 8.92 | 331.07 | 5.50 |
| 2 | 1.62 | 3.38 | 425.77 | 32.08 | |
| 4 | 1.12 | 4.87 | 334.18 | 35.55 | |
| - | 7 | 9.81 | 7.39 | 301.29 | 55.32 |
| - | 14 | 0.63 | 14.12 | 270.15 | 47.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Shi, Q.; Peng, R.; Sun, T.; Zhang, Z.; Li, L.; Xie, X. Effect of One Sulfate-Reducing Bacterium SRB-Z Isolated from Pearl River on the Corrosion Behavior of Q235 Carbon Steel. Coatings 2023, 13, 478. https://doi.org/10.3390/coatings13020478
Qi H, Shi Q, Peng R, Sun T, Zhang Z, Li L, Xie X. Effect of One Sulfate-Reducing Bacterium SRB-Z Isolated from Pearl River on the Corrosion Behavior of Q235 Carbon Steel. Coatings. 2023; 13(2):478. https://doi.org/10.3390/coatings13020478
Chicago/Turabian StyleQi, Hong, Qingshan Shi, Ruqun Peng, Tingli Sun, Zheng Zhang, Liangqiu Li, and Xiaobao Xie. 2023. "Effect of One Sulfate-Reducing Bacterium SRB-Z Isolated from Pearl River on the Corrosion Behavior of Q235 Carbon Steel" Coatings 13, no. 2: 478. https://doi.org/10.3390/coatings13020478
APA StyleQi, H., Shi, Q., Peng, R., Sun, T., Zhang, Z., Li, L., & Xie, X. (2023). Effect of One Sulfate-Reducing Bacterium SRB-Z Isolated from Pearl River on the Corrosion Behavior of Q235 Carbon Steel. Coatings, 13(2), 478. https://doi.org/10.3390/coatings13020478