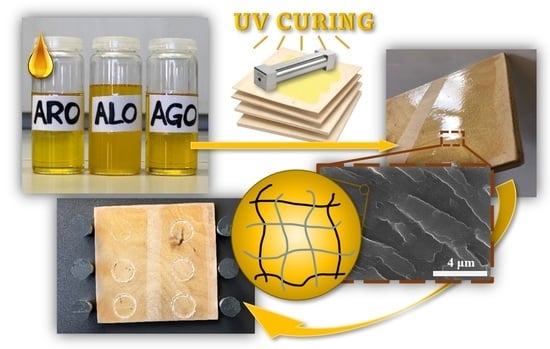
Tailored Biobased Resins from Acrylated Vegetable Oils for Application in Wood Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Curing Process of Coatings
2.3. Viscosity
2.4. Scanning Electron Microscopy
2.5. Dynamic Mechanical Analysis
2.6. Tensile Tests
2.7. Pull-Off Adhesion Test
2.8. Photo-Oxidation
2.9. Microhardness
2.10. Sliding Friction Test
3. Results and Discussion
3.1. Physical Properties
3.2. SEM Structure Studies
3.3. Dynamic Mechanical Properties
3.4. Tensile Properties
3.5. Adhesion Strength
3.6. Photo-Oxidation Effect on Coatings’ Properties
3.7. Sliding Friction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, M.F.; Campbell, J.D.; Fu, Z.; Bohling, J.; Leroux, J.G.; Mabee, W.; Robert, T. Future green chemistry and sustainability needs in polymeric coatings. Green Chem. 2019, 21, 4919–4926. [Google Scholar] [CrossRef]
- Valet, A. Outdoor applications of UV curable clearcoats-a real alternative to thermally cured clearcoats. Prog. Org. Coat. 1999, 35, 223–233. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B.; Wang, W.; Li, C.R.; Wang, Q. Use of vegetable oils as transformer oils–a review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Wang, R.; Schuman, T.P. Vegetable oil-derived epoxy monomers and polymer blends: A comparative study with review. Express Polym. Lett. 2013, 7, 272–292. [Google Scholar] [CrossRef]
- Mendoza, G.; Igartua, A.; Fernandez-Diaz, B.; Urquiola, F.; Vivanco, S.; Arguizoniz, R. Vegetable oils as hydraulic fluids for agricultural applications. Grasas Y Aceites 2011, 62, 29–38. [Google Scholar]
- Briede, S.; Jurinovs, M.; Nechausov, S.; Platnieks, O.; Gaidukovs, S. State-of-the-art UV-assisted 3D printing via a rapid syringe-extrusion approach for photoactive vegetable oil acrylates produced in one-step synthesis. Mol. Syst. Des. Eng. 2022, 7, 1434–1448. [Google Scholar] [CrossRef]
- Wuzella, G.; Mahendran, A.R.; Müller, U.; Kandelbauer, A.; Teischinger, A. Photocrosslinking of an Acrylated Epoxidized Linseed Oil: Kinetics and its Application for Optimized Wood Coatings. J. Polym. Environ. 2012, 20, 1063–1074. [Google Scholar] [CrossRef]
- Black, M.; Rawlins, J.W. Thiol–ene UV-curable coatings using vegetable oil macromonomers. Eur. Polym. J. 2009, 45, 1433–1441. [Google Scholar] [CrossRef]
- Thanamongkollit, N.; Miller, K.R.; Soucek, M.D. Synthesis of UV-curable tung oil and UV-curable tung oil based alkyd. Prog. Org. Coat. 2012, 73, 425–434. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Abdullah, L.C.; Rayung, M.; Li, W.J. A comparative study of acrylate oligomer on Jatropha and Palm oil-based UV-curable surface coating. Ind. Crops Prod. 2015, 77, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Grüneberger, F.; Künniger, T.; Zimmermann, T.; Arnold, M. Rheology of nanofibrillated cellulose/acrylate systems for coating applications. Cellulose 2014, 21, 1313–1326. [Google Scholar] [CrossRef]
- Wang, X.; Soucek, M.D. Investigation of non-isocyanate urethane dimethacrylate reactive diluents for UV-curable polyurethane coatings. Prog. Org. Coat. 2013, 76, 1057–1067. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Zhang, R.; Zhu, J.; Jiang, Y. Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: The role of rosin acid derivatives. Green Chem. 2013, 15, 1300–1310. [Google Scholar] [CrossRef]
- Rengasamy, S.; Mannari, V. Development of soy-based UV-curable acrylate oligomers and study of their film properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Liu, H.; Lu, W.; Liu, S. Development of acrylated soybean oil-based UV-curable coatings with high impact strength from low viscosity oligomer. J. Appl. Polym. Sci. 2018, 135, 45698. [Google Scholar] [CrossRef]
- Fei, M.-E.; Liu, W.; Jia, A.; Ban, Y.; Qiu, R. Bamboo fibers composites based on styrene-free soybean-oil thermosets using methacrylates as reactive diluents. Compos. Part A Appl. Sci. Manuf. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Xie, B.; Yang, Y.; Hu, Y.; Zhou, X.; Yuan, T.; Yang, Z. Bio-based polyfunctional reactive diluent derived from tung oil by thiol-ene click reaction for high bio-content UV-LED curable coatings. Ind. Crops Prod. 2021, 160, 113117. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, L.; Gao, Z.; Kessler, M.R. Synthesis and Characterization of Methacrylated Eugenol as a Sustainable Reactive Diluent for a Maleinated Acrylated Epoxidized Soybean Oil Resin. ACS Sustain. Chem. Eng. 2017, 5, 8876–8883. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783. [Google Scholar] [CrossRef]
- Dai, J.; Liu, X.; Ma, S.; Wang, J.; Shen, X.; You, S.; Zhu, J. Soybean oil-based UV-curable coatings strengthened by crosslink agent derived from itaconic acid together with 2-hydroxyethyl methacrylate phosphate. Prog. Org. Coat. 2016, 97, 210–215. [Google Scholar] [CrossRef]
- Williams, R.J.J.; Rozenberg, B.A.; Pascault, J.-P. Reaction-induced phase separation in modified thermosetting polymers. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2005; pp. 95–156. [Google Scholar]
- Mirzaee Ghazani, S.; Garcia-Llatas, G.; Marangoni, A. Micronutrient content of cold-pressed, hot-pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Technol. 2014, 116, 380–387. [Google Scholar] [CrossRef]
- Taherkhani, M.; Hashemzadeh Gargari, M.; Sadrameli, S.M. Investigating the Batch and Continuous Biodiesel Production from Linseed Oil in the Presence of a Heterogeneous Based Catalyst in a Packed Bed Reactor. J. Renew. Energy Environ. 2018, 148, 888–895. [Google Scholar]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Olivier, A.; Benkhaled, L.; Pakula, T.; Ewen, B.; Best, A.; Benmouna, M.; Maschke, U. Static and Dynamic Mechanical Behavior of Electron Beam-Cured Monomer and Monomer/Liquid Crystal Systems. Macromol. Mater. Eng. 2004, 289, 1047–1052. [Google Scholar] [CrossRef]
- Taki, K.; Watanabe, Y.; Ito, H.; Ohshima, M. Effect of oxygen inhibition on the kinetic constants of the UV-radical photopolymerization of diurethane dimethacrylate/photoinitiator systems. Macromolecules 2014, 47, 1906–1913. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Wardana, I.; Widodo, A.; Wijayanti, W. Improving vegetable oil properties by transforming fatty acid chain length in jatropha oil and coconut oil blends. Energies 2018, 11, 394. [Google Scholar]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crops Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Zong, Z.; Soucek, M.D.; Liu, Y.; Hu, J. Cationic photopolymerization of epoxynorbornane linseed oils: The effect of diluents. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3440–3456. [Google Scholar] [CrossRef]
- Anastasio, R.; Cardinaels, R.; Peters, G.W.M.; Breemen, L.C.A. Structure–mechanical property relationships in acrylate networks. J. Appl. Polym. Sci. 2019, 137, 48498. [Google Scholar] [CrossRef]
- Campanella, A.; La Scala, J.J.; Wool, R.P. The use of acrylated fatty acid methyl esters as styrene replacements in triglyceride-based thermosetting polymers. Polym. Eng. Sci. 2009, 49, 2384–2392. [Google Scholar] [CrossRef]
- LaScala, J.J.; Jeyarajasingam, A.; Winston, C.; Sand, J.M.; Palmese, G.R. Predicting the Viscosity of Low VOC Vinyl Ester and Fatty Acid-Based Resins; U.S. Army Research Laboratory, Aberdeen Proving Ground: Aberdeen Proving Ground, MD, USA, 2005. [Google Scholar]
- Worthington, K.S.; Baguenard, C.; Forney, B.S.; Guymon, C.A. Photopolymerization kinetics in and of self-assembling lyotropic liquid crystal templates. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 471–489. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, Z.; Moon, K.-S.; Wong, C.P. Glass transition and relaxation behavior of epoxy nanocomposites. J. Polym. Sci. Part B: Polym. Phys. 2004, 42, 3849–3858. [Google Scholar] [CrossRef]
- Hazarika, A.; Mandal, M.; Maji, T.K. Dynamic mechanical analysis, biodegradability and thermal stability of wood polymer nanocomposites. Compos. Part B Eng. 2014, 60, 568–576. [Google Scholar] [CrossRef]
- Núñez-Regueira, L.; Villanueva, M.; Fraga-Rivas, I. Effect of a reactive diluent on the curing and dynamomechanical properties of an epoxy-diamine system. J. Therm. Anal. Calorim. 2006, 86, 463–468. [Google Scholar] [CrossRef]
- Henna, P.H.; Andjelkovic, D.D.; Kundu, P.P.; Larock, R.C. Biobased thermosets from the free-radical copolymerization of conjugated linseed oil. J. Appl. Polym. Sci. 2007, 104, 979–985. [Google Scholar] [CrossRef]
- Lu, J.; Khot, S.; Wool, R.P. New sheet molding compound resins from soybean oil. I. Synthesis and characterization. Polymer 2005, 46, 71–80. [Google Scholar] [CrossRef]
- Yin, Y.; Yao, S.; Zhou, X. Synthesis and dynamic mechanical behavior of crosslinked copolymers and IPNs from vegetable oils. J. Appl. Polym. Sci. 2003, 88, 1840–1842. [Google Scholar] [CrossRef]
- Coran, A.Y. Chapter 7–Vulcanization. In The Science and Technology of Rubber, 4th ed.; Mark, J.E., Erman, B., Roland, C.M., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 337–381. [Google Scholar]
- Li, F.; Larock, R.C. New soybean oil-styrene-divinylbenzene thermosetting copolymers. III. Tensile stress-strain behavior. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 60–77. [Google Scholar] [CrossRef]
- Zhou, L.M.; Yeung, K.W.; Yuen, C.W.M.; Zhou, X. Tensile Strength Loss of Mercerized and Crosslinked Ramie Fabric. Text. Res. J. 2003, 74, 367–372. [Google Scholar] [CrossRef]
- De Godoy Fróes-Salgado, N.R.; Gajewski, V.; Ornaghi, B.P.; Pfeifer, C.S.C.; Meier, M.M.; Xavier, T.A.; Braga, R.R. Influence of the base and diluent monomer on network characteristics and mechanical properties of neat resin and composite materials. Odontology 2014, 103, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Duan, Q.; Zhu, J.; Liu, H.; Chen, L.; Yu, L. Anchor and bridge functions of APTES layer on interface between hydrophilic starch films and hydrophobic soyabean oil coating. Carbohydr. Polym. 2021, 272, 118450. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S.; Robinson, P.J.; White, N.J.; Swales, D.W. Photo-oxidative stability of electron-beam and UV cured triacrylate resin films: Influence of the structure of the multifunctional monomer. Polym. Degrad. Stab. 1989, 23, 245–255. [Google Scholar] [CrossRef]
- Dupuis, A.; Perrin, F.-X.; Ulloa Torres, A.; Habas, J.-P.; Belec, L.; Chailan, J.-F. Photo-oxidative degradation behavior of linseed oil based epoxy resin. Polym. Degrad. Stab. 2017, 135, 73–84. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Epoxidized soybean oil/ZnO biocomposites for soft tissue applications: Preparation and characterization. ACS Appl. Mater. Interfaces 2014, 6, 17277–17288. [Google Scholar] [CrossRef]
- Rahman, M.S.B.A.; Shaktur, K.M.; Mohammad, R.; Zalikha, W.A.; Nawi, N.; Mohd, A.F. Optical properties and indentation hardness of thin-film acrylated epoxidized oil. Opt. Eng. 2012, 51, 025002. [Google Scholar] [CrossRef]
- Ivakina, K.; Skadins, E.; Kiyanitsa, A.; Gaidukov, S.; Tupureina, V.; Cabulis, U.; Maksimov, R.D. Influence of nanoclay additive on mechanical properties of bio-based polyurethane nanocomposites. Proc. Key Eng. Mater. 2013, 559, 37–42. [Google Scholar] [CrossRef]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T. Crosslinker copolymerization for property control in inverse vulcanization. Chem.–A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef]
- Zamfirova, G.; Lorenzo, V.; Benavente, R.; Pereña, J.M. On the relationship between modulus of elasticity and microhardness. J. Appl. Polym. Sci. 2003, 88, 1794–1798. [Google Scholar] [CrossRef]
- Evans, G.; Behiri, J.; Currey, J.; Bonfield, W. Microhardness and Young’s modulus in cortical bone exhibiting a wide range of mineral volume fractions, and in a bone analogue. J. Mater. Sci. Mater. Med. 1990, 1, 38–43. [Google Scholar] [CrossRef]
- Persson, B.N.J. Sliding Friction: Physical Principles and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bexell, U.; Olsson, M.; Johansson, M.; Samuelsson, J.; Sundell, P.-E. A tribological study of a novel pre-treatment with linseed oil bonded to mercaptosilane treated aluminium. Surf. Coat. Technol. 2003, 166, 141–152. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhang, Y.; Li, B.; Yang, M.; Zhang, X.; Guo, S.; Liu, G.; Zhai, M. Comparative evaluation of the lubricating properties of vegetable-oil-based nanofluids between frictional test and grinding experiment. J. Manuf. Process. 2017, 26, 94–104. [Google Scholar] [CrossRef]
- Bikerman, J.J. Sliding friction of polymers. J. Macromol. Sci. Part C Polym. Rev. 1974, 11, 1–44. [Google Scholar] [CrossRef]








| Biobased Resins | Resin Composition | Monomers (wt%) | |||
|---|---|---|---|---|---|
| R | L | G | GPT | ||
| RL | R/L (50/50) | 50 | 50 | 0 | 0 |
| RG | R/G (50/50) | 50 | 0 | 50 | 0 |
| LG | L/G (50/50) | 0 | 50 | 50 | 0 |
| RL_G5 | R/L (50/50) 95 wt%, GPT 5 wt% | 47.5 | 47.5 | 0 | 5 |
| RG_G5 | R/G (50/50) 95 wt%, GPT 5 wt% | 47.5 | 0 | 47.5 | 5 |
| LG_G5 | L/G (50/50) 95 wt%, GPT 5 wt% | 0 | 47.5 | 47.5 | 5 |
| RL_G20 | R/L (50/50) 80 wt%, GPT 20 wt% | 40 | 40 | 0 | 20 |
| RG_G20 | R/G (50/50) 80 wt%, GPT 20 wt% | 40 | 0 | 40 | 20 |
| LG_G20 | L/G (50/50) 80 wt%, GPT 20 wt% | 0 | 40 | 40 | 20 |
| Liquid | GPT Solubility | |
|---|---|---|
| Increased polarity  | Rapeseed oil | Insoluble |
| AVOs | Soluble | |
| Ethanol | Soluble | |
| Methanol | Soluble | |
| Water | Insoluble |
| Coatings | Tg (°C) 1 | E′ at Rubbery Plateau (MPa) | νe (mol/m3) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| RL | −34.0 | 13 | 1.5 | 0.70 ± 0.10 | 10.7 ± 1.5 |
| RG | −40.6 | 8 | 1.1 | 0.52 ± 0.08 | 6.3 ± 1.0 |
| LG | −37.6 | 7 | 0.7 | 0.48 ± 0.02 | 8.1 ± 1.1 |
| RL_G5 | −42.3 | 7 | 1.0 | 0.27 ± 0.06 | 2.7 ± 0.5 |
| RG_G5 | −31.2 | 13 | 1.7 | 0.34 ± 0.05 | 3.8 ± 0.4 |
| LG_G5 | −24.7 | 11 | 1.4 | 0.20 ± 0.04 | 2.2 ± 0.2 |
| RL_G20 | −31.3 | 17 | 2.1 | 0.76 ± 0.10 | 5.3 ± 0.6 |
| RG_G20 | −30.4 | 17 | 2.2 | 0.64 ± 0.16 | 3.5 ± 0.1 |
| LG_G20 | −32.1 | 13 | 1.9 | 0.67 ± 0.12 | 4.1 ± 0.1 |
| Coatings | Average Adhesion Strength (MPa) | Type of Failure |
|---|---|---|
| RL | 0.56 ± 0.12 | Glue failure |
| RL_G5 | 0.87 ± 0.15 | Mixed (adhesive + cohesive failure) |
| RL_G20 | 1.21 ± 0.14 | Mixed (adhesive + cohesive failure) |
| Photo-Oxidized (9 h) Coatings | μ (in Plateau Zone) | ||
|---|---|---|---|
| 0.5 (N) | 1.5 (N) | 3.0 (N) | |
| RL | 0.24 | 0.88 | 0.79 |
| RL_G5 | 0.42 | 0.65 | 0.80 |
| RL_G20 | 0.21 | 0.47 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briede, S.; Platnieks, O.; Barkane, A.; Sivacovs, I.; Leitans, A.; Lungevics, J.; Gaidukovs, S. Tailored Biobased Resins from Acrylated Vegetable Oils for Application in Wood Coatings. Coatings 2023, 13, 657. https://doi.org/10.3390/coatings13030657
Briede S, Platnieks O, Barkane A, Sivacovs I, Leitans A, Lungevics J, Gaidukovs S. Tailored Biobased Resins from Acrylated Vegetable Oils for Application in Wood Coatings. Coatings. 2023; 13(3):657. https://doi.org/10.3390/coatings13030657
Chicago/Turabian StyleBriede, Sabine, Oskars Platnieks, Anda Barkane, Igors Sivacovs, Armands Leitans, Janis Lungevics, and Sergejs Gaidukovs. 2023. "Tailored Biobased Resins from Acrylated Vegetable Oils for Application in Wood Coatings" Coatings 13, no. 3: 657. https://doi.org/10.3390/coatings13030657
APA StyleBriede, S., Platnieks, O., Barkane, A., Sivacovs, I., Leitans, A., Lungevics, J., & Gaidukovs, S. (2023). Tailored Biobased Resins from Acrylated Vegetable Oils for Application in Wood Coatings. Coatings, 13(3), 657. https://doi.org/10.3390/coatings13030657