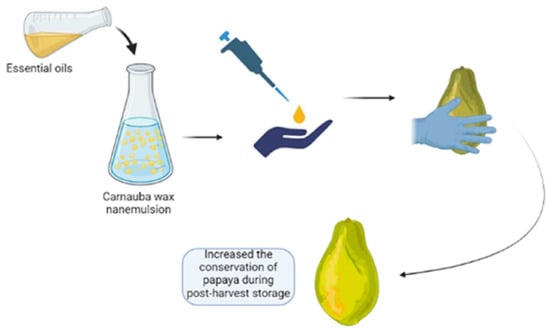
Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Essential Oil Composition
2.3. Encapsulation of Essential Oils with β-Cyclodextrin
2.4. Edible Coating Preparation
2.5. Physicochemical Parameters of Papayas
2.6. Respiration Rate and Ethylene of Papayas
2.7. Scanning Electron Microscopy
2.8. Decay Percentage and Severity on Papayas
2.9. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Composition
3.2. Physicochemical Parameters of Papayas
3.3. Scanning Electron Microscopy
3.4. Decay Percentage and Severity on Papayas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial spoilage of vegetables, fruits and cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2022, 375, 131810. [Google Scholar] [CrossRef] [PubMed]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: Recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2020, 62, 1912–1935. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Wu, S.; Siddiqui, M.W. Incorporating essential oils or compounds derived thereof into edible coatings: Effect on quality and shelf life of fresh/fresh-cut produce. Trends Food Sci. Technol. 2021, 108, 245–257. [Google Scholar] [CrossRef]
- Miranda, M.; Ribeiro, M.D.M.M.; Spricigo, P.C.; Pilon, L.; Mitsuyuki, M.C.; Correa, D.S.; Ferreira, M.D. Carnauba wax nanoemulsion applied as an edible coating on fresh tomato for postharvest quality evaluation. Heliyon 2022, 8, e09803. [Google Scholar] [CrossRef]
- Yousuf, B.; Sun, Y.; Wu, S. Lipid and Lipid-containing Composite Edible Coatings and Films. Food Rev. Int. 2021, 38, 574–597. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Hasnain, A. Mango kernel starch as a novel edible coating for enhancing shelf- life of tomato (Solanum lycopersicum) fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. [Google Scholar] [CrossRef]
- Susmita Devi, L.; Kalita, S.; Mukherjee, A.; Kumar, S. Carnauba wax-based composite films and coatings: Recent advancement in prolonging postharvest shelf-life of fruits and vegetables. Trends Food Sci. Technol. 2022, 129, 296–305. [Google Scholar] [CrossRef]
- de Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. Carnauba wax uses in food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef]
- Filho, J.G.D.O.; Silva, G.D.C.; Oldoni, F.C.A.; Miranda, M.; Florencio, C.; de Oliveira, R.M.D.; Gomes, M.D.P.; Ferreira, M.D. Edible Coating Based on Carnauba Wax Nanoemulsion and Cymbopogon martinii Essential Oil on Papaya Postharvest Preservation. Coatings 2022, 12, 1700. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Bezerra, C.C.D.O.N.; Albiero, B.R.; Oldoni, F.C.A.; Miranda, M.; Egea, M.B.; de Azeredo, H.M.C.; Ferreira, M.D. New approach in the development of edible films: The use of carnauba wax micro- or nanoemulsions in arrowroot starch-based films. Food Packag. Shelf Life 2020, 26, 100589. [Google Scholar] [CrossRef]
- de Castro e Silva, P.; de Oliveira, A.C.S.; Pereira, L.A.S.; Valquíria, M.; Carvalho, G.R.; Miranda, K.W.E.; Marconcini, J.M.; Oliveira, J.E. Development of bionanocomposites of pectin and nanoemulsions of carnauba wax and neem oil pectin/carnauba wax/neem oil composites. Polym. Compos. 2020, 41, 858–870. [Google Scholar] [CrossRef]
- Mayer, S.; Weiss, J.; McClements, D.J. Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: Factors influencing droplet size and stability. J. Colloid Interface Sci. 2013, 402, 122–130. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- McClements, D.J. Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 2011, 7, 2297–2316. [Google Scholar] [CrossRef] [Green Version]
- Zucchini, N.M.; Florencio, C.; Miranda, M.; Borba, K.R.; Oldoni, F.C.A.; Oliveira Filho, J.G.; Bonfim, N.S.; Rodrigues, K.A.; de Oliveira, R.M.D.; Mitsuyuki, M.C.; et al. Effect of carnauba wax nanoemulsion coating on postharvest papaya quality. Acta Hortic. 2021, 1325, 199–206. [Google Scholar] [CrossRef]
- Yan, J.; Wu, H.; Shi, F.; Wang, H.; Chen, K.; Feng, J.; Jia, W. Antifungal activity screening for mint and thyme essential oils against Rhizopus stolonifer and their application in postharvest preservation of strawberry and peach fruits. J. Appl. Microbiol. 2021, 130, 1993–2007. [Google Scholar] [CrossRef]
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2021, 62, 5029–5055. [Google Scholar] [CrossRef]
- Pandey, V.K.; Islam, R.U.; Shams, R.; Dar, A.H. A comprehensive review on the application of essential oils as bioactive compounds in Nano-emulsion based edible coatings of fruits and vegetables. Appl. Food Res. 2022, 2, 100042. [Google Scholar] [CrossRef]
- Fonseca, J.D.M.; Trevisol, T.C.; Valencia, G.A.; Monteiro, A.R. Recent trends in composite nanoemulsions for food packaging applications. In Bio-Based Nanoemulsions Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 387–398. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Liu, L.; Liu, Y.; Niu, X. Incorporation of clove essential oil nanoemulsion in chitosan coating to control Burkholderia gladioli and improve postharvest quality of fresh Tremella fuciformis. LWT 2022, 170, 114059. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007, 100, 451–458. [Google Scholar] [CrossRef]
- Sarkhosh, A.; Schaffer, B.; Vargas, A.I.; Palmateer, A.J.; Lopez, P.; Soleymani, A.; Farzaneh, M. Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol. Agric. Hortic. 2017, 34, 18–26. [Google Scholar] [CrossRef]
- Aminifard, M.H.; Mohammadi, S. Essential oils to control Botrytis cinerea in vitro and in vivo on plum fruits. J. Sci. Food Agric. 2013, 93, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.G.R.; Ferreira, N.C.A.; Fiocco, A.C.T.R.; Picone, C.S.F. Lactoferrin-Chitosan-TPP Nanoparticles: Antibacterial Action and Extension of Strawberry Shelf-Life. Food Bioprocess Technol. 2023, 16, 135–148. [Google Scholar] [CrossRef]
- Duarte, L.G.R. Natural antimicrobials: Healthy alternative of application in foods as preservatives. Int. J. Health Sci. 2022, 2. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [Green Version]
- Sumalan, R.M.; Kuganov, R.; Obistioiu, D.; Popescu, I.; Radulov, I.; Alexa, E.; Negrea, M.; Salimzoda, A.F.; Sumalan, R.L.; Cocan, I. Assessment of Mint, Basil, and Lavender Essential Oil Vapor-Phase in Antifungal Protection and Lemon Fruit Quality. Molecules 2020, 25, 1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago, C.M.L.; Artiga-Artigas, M.; Antunes, M.D.C.; Faleiro, M.L.; Miguel, M.G.; Martín-Belloso, O. Effectiveness of nanoemulsions of clove and lemongrass essential oils and their major components against Escherichia coli and Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2721–2736. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, D.R. Nanoemulsions based edible coatings with potential food applications. Int. J. Biobased Plast. 2021, 3, 112–125. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [Green Version]
- Zamaniahari, S.; Jamshidi, A.; Moosavy, M.H.; Khatibi, S.A. Preparation and evaluation of Mentha spicata L. essential oil nanoemulsion: Physicochemical properties, antibacterial activity against foodborne pathogens and antioxidant properties. J. Food Meas. Charact. 2022, 16, 3289–3300. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Santamaría Basulto, F.; Sauri Duch, E.; Espadas y Gil, F.; Díaz Plaza, R.; Larqué Saavedra, A.; Santamaría, J.M. Postharvest ripening and maturity indices for maradol papaya. Interciencia 2009, 34, 583–588. [Google Scholar]
- Viacava, G.E.; Ayala-Zavala, J.F.; González-Aguilar, G.A.; Ansorena, M.R. Effect of free and microencapsulated thyme essential oil on quality attributes of minimally processed lettuce. Postharvest Biol. Technol. 2018, 145, 125–133. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Baker, R.A. Edible Coatings from Morpholine-Free Wax Microemulsions. J. Agric. Food Chem. 1997, 45, 349–352. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Ference, C.; Plotto, A.; Bai, J.; Wood, D.; Assis, O.B.G.; Ferreira, M.D.; Baldwin, E. Nano- and Micro- Carnauba Wax Emulsions versus Shellac Protective Coatings on Postharvest Citrus Quality. J. Am. Soc. Hortic. Sci. 2021, 146, 40–49. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Method 935.14 and 992.24; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Martins, D.R.; Barbosa, N.C.; de Resende, E.D. Respiration rate of Golden papaya stored under refrigeration and with different controlled atmospheres. Sci. Agric. 2014, 71, 369–373. [Google Scholar] [CrossRef]
- Miranda, M.; Sun, X.; Marín, A.; dos Santos, L.C.; Plotto, A.; Bai, J.; Benedito Garrido Assis, O.; David Ferreira, M.; Baldwin, E. Nano- and micro-sized carnauba wax emulsions-based coatings incorporated with ginger essential oil and hydroxypropyl methylcellulose on papaya: Preservation of quality and delay of post-harvest fruit decay. Food Chem. 2022, 3, 100249. [Google Scholar] [CrossRef] [PubMed]
- Kamal, I.; Khedr, A.I.M.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Saad, A.S. Chemotherapeutic and chemopreventive potentials of ρ-coumaric acid—Squid chitosan nanogel loaded with Syzygium aromaticum essential oil. Int. J. Biol. Macromol. 2021, 188, 523–533. [Google Scholar] [CrossRef]
- Ferreira, V.R.F.; Brandão, R.M.; Freitas, M.P.; Saczk, A.A.; Felix, F.S.; Silla, J.M.; Teixeira, M.L.; Cardoso, M.D.G. Colorimetric, electroanalytical and theoretical evaluation of the antioxidant activity of Syzygium aromaticum L.; Origanum vulgare L.; Mentha spicata L. and Eremanthus erythropappus M. essential oils, and their major constituents. New J. Chem. 2019, 43, 7653–7662. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia island (Italy). Nat. Prod. Res. 2021, 35, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, C.S.; Chauhan, N.; Dolma, S.K.; Reddy, S.G.E. Chemical Composition and Insecticidal Activities of Essential Oils against the Pulse Beetle. Molecules 2022, 27, 568. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [Green Version]
- Saadat, S.; Rawtani, D.; Rao, P.K. Antibacterial activity of chitosan film containing Syzygium aromaticum (clove) oil encapsulated halloysite nanotubes against foodborne pathogenic bacterial strains. Mater. Today Commun. 2022, 32, 104132. [Google Scholar] [CrossRef]
- Santolin, L.; Fiametti, K.G.; da Silva Lobo, V.; Wancura, J.H.C.; Oliveira, J.V. Enzymatic Synthesis of Eugenyl Acetate from Essential Oil of Clove Using Lipases in Liquid Formulation as Biocatalyst. Appl. Biochem. Biotechnol. 2021, 193, 3512–3527. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal Activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa Essential Oils against Human Pathogenic Fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. [Google Scholar] [CrossRef]
- Kostik, V.; Gjorgjeska, B.; Petkovska, S.; Mentha, L. essential oils composition and in vitro antifungal activity. IOSR J. Pharm. 2015, 5, 1–7. [Google Scholar]
- Duarte, L.G.R.; Alencar, W.M.P.; Iacuzio, R.; Silva, N.C.C.; Picone, C.S.F. Synthesis, characterization and application of antibacterial lactoferrin nanoparticles. Curr. Res. Food Sci. 2022, 5, 642–652. [Google Scholar] [CrossRef]
- Luksiene, Z.; Buchovec, I. Impact of chlorophyllin-chitosan coating and visible light on the microbial contamination, shelf life, nutritional and visual quality of strawberries. Innov. Food Sci. Emerg. Technol. 2019, 52, 463–472. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, D.; Ren, D.; Zeng, K.; Wu, X. Green synthesis of zinc oxide nanoparticles using Citrus sinensis peel extract and application to strawberry preservation: A comparison study. LWT 2020, 126, 109297. [Google Scholar] [CrossRef]
- Gutiérrez-Pacheco, M.M.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Miranda, R.; Ayala-Zavala, J.F. Individual and Combined Coatings of Chitosan and Carnauba Wax with Oregano Essential Oil to Avoid Water Loss and Microbial Decay of Fresh Cucumber. Coatings 2020, 10, 614. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Guo, X.; Qin, Y.; Shen, P.; Peng, Q. A Novel Sodium Alginate-Carnauba Wax Film Containing Calcium Ascorbate: Structural Properties and Preservative Effect on Fresh-Cut Apples. Molecules 2023, 28, 367. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Burns, J.K.; Kazokas, W.; Brecht, J.K.; Hagenmaier, R.D.; Bender, R.J.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol. Technol. 1999, 17, 215–226. [Google Scholar] [CrossRef]
- Mendy, T.K.; Misran, A.; Mahmud, T.M.M.; Ismail, S.I. Application of Aloe vera coating delays ripening and extend the shelf life of papaya fruit. Sci. Hortic. 2019, 246, 769–776. [Google Scholar] [CrossRef]
- Sekarina, A.S.; Supriyadi; Munawaroh, H.S.H.; Susanto, E.; Show, P.L.; Ningrum, A. Effects of edible coatings of chitosan—Fish skin gelatine containing black tea extract on quality of minimally processed papaya during refrigerated storage. Carbohydr. Polym. Technol. Appl. 2023, 5, 100287. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Picone, C.S.F. Antimicrobial activity of lactoferrin-chitosan-gellan nanoparticles and their influence on strawberry preservation. Food Res. Int. 2022, 159, 111586. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yu, L.; Hu, Y.; Zhu, Z.; Zhuang, C.; Zhao, Y.; Zhong, Y. Electrostatic spraying of chitosan coating with different deacetylation degree for strawberry preservation. Int. J. Biol. Macromol. 2019, 139, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Khan, M.A. Modified atmosphere packaging of fresh-cut papaya using alginate based edible coating: Quality evaluation and shelf life study. Sci. Hortic. 2020, 259, 108853. [Google Scholar] [CrossRef]
- Vieira, A.C.F.; de Matos Fonseca, J.; Menezes, N.M.C.; Monteiro, A.R.; Valencia, G.A. Active coatings based on hydroxypropyl methylcellulose and silver nanoparticles to extend the papaya (Carica papaya L.) shelf life. Int. J. Biol. Macromol. 2020, 164, 489–498. [Google Scholar] [CrossRef]
- Malvano, F.; Corona, O.; Pham, P.L.; Cinquanta, L.; Pollon, M.; Bambina, P.; Farina, V.; Albanese, D. Effect of alginate-based coating charged with hydroxyapatite and quercetin on colour, firmness, sugars and volatile compounds of fresh cut papaya during cold storage. Eur. Food Res. Technol. 2022, 248, 2833–2842. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Amin Tawakkal, I.S.M.; Muda Mohamed, M.T.; Hazwan Hamzah, M.; Ali, M.M.; Abd Razak, M.F.H. Kinetics of quality changes in papayas (Carica papaya L.) coated with Malaysian stingless bee honey. Sci. Hortic. 2020, 267, 109321. [Google Scholar] [CrossRef]
- Nazoori, F.; Mollai, S.; Sobhani, F.; Mirdehghan, S.H.; Sahhafi, S.R. Carboxymethyl cellulose and carnauba wax treatments kept the pomegranate fruit (Punica granatum L.) quality during cold storage via improving enzymatic defense system and bioactive compounds. Sci. Hortic. 2023, 309, 111645. [Google Scholar] [CrossRef]
- Tan, G.H.; Ali, A.; Siddiqui, Y. Current strategies, perspectives and challenges in management and control of postharvest diseases of papaya. Sci. Hortic. 2022, 301, 111139. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Edible alginate-based coating as carrier of antimicrobials to improve shelf-life and safety of fresh-cut melon. Int. J. Food Microbiol. 2008, 121, 313–327. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Z.; Yang, H. Effect of carnauba wax-based coating containing glycerol monolaurate on the quality maintenance and shelf-life of Indian jujube (Zizyphus mauritiana Lamk.) fruit during storage. Sci. Hortic. 2019, 244, 157–164. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, M.; Liu, M.; Su, D.; Chen, J.; Gao, Y.; Bouzayen, M.; Li, Z. The Molecular Regulation of Ethylene in Fruit Ripening. Small Methods 2020, 4, 1900485. [Google Scholar] [CrossRef]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). LWT 2016, 70, 213–222. [Google Scholar] [CrossRef]
- Özden, Ç.; Bayindirli, L. Effects of combinational use of controlled atmosphere, cold storage and edible coating applications on shelf life and quality attributes of green peppers. European Food Res. Technol. 2002, 214, 320–326. [Google Scholar] [CrossRef]
- Boshkova, N.; Stambolova, I.; Stoyanova, D.; Simeonova, S.; Grozev, N.; Avdeev, G.; Shipochka, M.; Dimitrov, O.; Bachvarov, V.; Peshova, M.; et al. Protective Characteristics of TiO2 Sol-Gel Layer Deposited on Zn-Ni or Zn-Co Substrates. Coatings 2023, 13, 295. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll. 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; McClements, D.J. Fabrication and stability of colloidal delivery systems for flavor oils: Effect of composition and storage conditions. Food Res. Int. 2012, 46, 209–216. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Longaray Delamare, A.P.; Fernandes Pauletti, G.; Barcellos, T. Poly(lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 326, 126997. [Google Scholar] [CrossRef]
- Vellido-Perez, J.A.; Ochando-Pulido, J.M.; Brito-de la Fuente, E.; Martinez-Ferez, A. Novel emulsions–based technological approaches for the protection of omega–3 polyunsaturated fatty acids against oxidation processes—A comprehensive review. Food Struct. 2021, 27, 100175. [Google Scholar] [CrossRef]




| Compound | Syzigium aromaticum (% Area) | Mentha spicata (% Area) |
|---|---|---|
| α-Pinene | - | 0.69 |
| Sabinene | - | 0.32 |
| β-Pinene | - | 0.76 |
| Myrcene | - | 0.95 |
| 3-Octanol | - | 0.25 |
| p-Cymene | - | 0.23 |
| Limonene | - | 20.34 |
| 1,8-Cineol | - | 1.10 |
| γ-Terpinene | - | 0.13 |
| Menthone | - | 0.50 |
| cis-Sabinene hydrate | - | 0.17 |
| Menthol | - | 0.15 |
| Isomenthol | - | 1.06 |
| (E)-dihydrocarvone | - | 1.40 |
| cis-Dihydrocarvone | - | 0.15 |
| trans-Carveol | - | 0.28 |
| Carvone | - | 68.88 |
| Piperitone | - | 0.18 |
| Menthyl acetate | - | 0.39 |
| Dihydrocarvyl acetate | - | 0.13 |
| Eugenol | 89.73 | - |
| cis-Carvyl acetate | - | 0.11 |
| β-bourbenene | - | 0.77 |
| β-Gurjenene | 7.59 | - |
| Caryophyllene | - | 1.03 |
| α-Humulene | 2.10 | - |
| γ-Selinene | 0.20 | - |
| δ-Cadinene | 0.25 | - |
| Caryophyllene oxide | 0.13 | - |
| Total | 100 | 99.97 |
| Treatments | pH | TSS (%) | TA (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Days) | 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 |
| Control | 5.35 ± 0.14 b | 5.68 ± 0.10 b | 6.05 ± 0.19 ab | 10.02 ± 1.44 a | 8.06 ± 1.45 b | 8.97 ± 2.31 a | 0.066 ± 0.013 bc | 0.097 ± 0.090 a | 0.055 ± 0.014 a |
| CWN | 5.64 ± 0.05 a | 6.00 ± 0.13 a | 6.28 ± 0.23 a | 8.59 ± 1.51 a | 8.87 ± 1.19 ab | 7.75 ± 1.32 a | 0.065 ± 0.009 c | 0.052 ± 0.007 a | 0.059 ± 0.010 a |
| CWN-CEO:β-CD | 5.48 ± 0.11 ab | 5.69 ± 0.14 b | 6.21 ± 0.14 ab | 8.76 ± 0.76 a | 9.43 ± 1.40 ab | 9.27 ± 1.99 a | 0.070 ± 0.010 bc | 0.057 ± 0.005 a | 0.051 ± 0.009 a |
| CWN-CEO | 5.49 ± 0.08 ab | 5.61 ± 0.21 b | 6.09 ± 0.12 ab | 8.80 ± 0.87 a | 9.02 ± 0.67 ab | 7.43 ± 1.20 a | 0.085 ± 0.012 a | 0.056 ± 0.005 a | 0.050 ± 0.008 a |
| CWN-MEO:β-CD | 5.45 ± 0.10 ab | 5.54 ± 0.14 b | 6.11 ± 0.10 ab | 9.02 ± 0.70 a | 8.67 ± 0.81 b | 7.90 ± 1.11 a | 0.081 ± 0.009 ab | 0.051 ± 0.006 a | 0.059 ± 0.006 a |
| CWN-MEO | 5.46 ± 0.27 ab | 5.72 ± 0.21 b | 6.00 ± 0.24 b | 9.84 ± 1.24 a | 10.37 ± 1.17 a | 8.25 ± 1.90 a | 0.068 ± 0.004 bc | 0.048 ± 0.011 a | 0.056 ± 0.020 a |
| Treatments | Time (Days) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | ||||||||||||
| L* | (h°) | L* | (h°) | ΔE* | L* | (h°) | ΔE* | L* | (h°) | ΔE* | |||||
| Control | 54.85 ± 3.91 a | 39.00 ± 2.13 a | 102.92 ± 2.55 a | 61.48 ± 5.77 a | 49.53 ± 6.98 a | 101.74 ± 9.47 a | 13.73 ± 5.74 a | 53.76 ± 6.93 a | 27.00 ± 4.31 a | 88.83 ± 11.20 a | 16.60 ± 1.54 a | 47.52 ± 10.88 b | 22.55 ± 7.14 b | 74.59 ± 12.82 a | 24.31 ± 6.82 a |
| CWN | 52.00 ± 3.39 b | 37.43 ± 1.89 a | 103.70 ± 2.34 a | 49.37 ± 4.75 c | 23.73 ± 3.05 b | 102.44 ± 7.42 a | 14.55 ± 3.05 a | 52.53 ± 6.55 a | 25.92 ± 3.06 a | 96.70 ± 10.69 a | 14.32 ± 2.41 a | 50.84 ± 8.58 b | 24.85 ± 4.75 b | 85.66 ± 15.19 a | 18.45 ± 3.33 a |
| CWN-CEO:β-CD | 53.10 ± 3.40 ab | 37.63 ± 2.04 a | 104.08 ± 2.33 a | 53.28 ± 7.65 bc | 31.79 ± 11.88 b | 103.79 ± 9.23 a | 13.14 ± 6.30 a | 54.43 ± 6.51 a | 27.80 ± 3.47 a | 92.87 ± 10.63 a | 14.15 ± 2.78 a | 49.83 ± 10.22 b | 24.58 ± 4.24 b | 80.35 ± 14.02 a | 20.19 ± 3.78 a |
| CWN-CEO | 54.86 ± 4.94 a | 38.45 ± 3.75 a | 102.89 ± 2.97 a | 61.49 ± 4.76 a | 48.17 ± 5.11 a | 103.86 ± 5.61 a | 12.32 ± 5.16 a | 56.55 ± 6.08 a | 28.43 ± 3.84 a | 87.93 ± 8.81 a | 15.15 ± 2.54 a | 52.44 ± 10.35 ab | 26.21 ± 6.18 b | 74.64 ± 10.14 a | 21.59 ± 3.42 a |
| CWN-MEO:β-CD | 53.63 ± 4.10 ab | 37.11 ± 2.60 a | 103.82 ± 2.66 a | 53.96 ± 5.89 bc | 26.25 ± 3.31 b | 97.05 ± 30.40 ab | 12.17 ± 1.27 a | 58.54 ± 5.65 a | 30.05 ± 3.48 a | 86.57 ± 27.61 a | 14.17 ± 3.51 a | 58.01 ± 7.39 a | 31.75 ± 4.56 a | 71.07 ± 29.77 a | 21.28 ± 3.73 a |
| CWN-MEO | 55.16 ± 4.21 a | 39.71 ± 4.25 a | 101.21 ± 4.05 b | 54.38 ± 5.80 b | 26.69 ± 3.84 b | 94.43 ± 9.61 b | 14.48 ± 2.01 a | 57.84 ± 5.97 a | 29.38 ± 4.21 a | 87.37 ± 10.81 a | 15.03 ± 2.49 a | 50.84 ± 8.58 b | 24.85 ± 4.75 b | 85.66 ± 15.19 a | 20.63 ± 5.07 a |
| Treatments | Storage Time (Days) | ||
|---|---|---|---|
| 5 | 10 | 15 | |
| Control | 5.46 ± 2.22 a | 5.52 ± 3.07 a | 4.02 ± 3.07 ab |
| CWN | 4.32 ± 3.02 a | 3.68 ± 1.33 a | 4.68 ± 2.20 ab |
| CWN-CEO:β-CD | 5.53 ± 3.10 a | 4.34 ± 0.92 a | 3.87 ± 2.30 ab |
| CWN-CEO | 6.59 ± 3.79 a | 3.83 ± 1.04 a | 6.60 ± 2.41 a |
| CWN-MEO:β-CD | 5.65 ± 3.35 a | 7.12 ± 2.10 a | 4.71 ± 1.70 ab |
| CWN-MEO | 5.15 ± 2.98 a | 3.88 ± 0.32 a | 2.82 ± 0.58 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira Filho, J.G.d.; Duarte, L.G.R.; Silva, Y.B.B.; Milan, E.P.; Santos, H.V.; Moura, T.C.; Bandini, V.P.; Vitolano, L.E.S.; Nobre, J.J.C.; Moreira, C.T.; et al. Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils. Coatings 2023, 13, 847. https://doi.org/10.3390/coatings13050847
Oliveira Filho JGd, Duarte LGR, Silva YBB, Milan EP, Santos HV, Moura TC, Bandini VP, Vitolano LES, Nobre JJC, Moreira CT, et al. Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils. Coatings. 2023; 13(5):847. https://doi.org/10.3390/coatings13050847
Chicago/Turabian StyleOliveira Filho, Josemar Gonçalves de, Larissa G. R. Duarte, Yasmin B. B. Silva, Eduardo P. Milan, Higor V. Santos, Thaís C. Moura, Vitor P. Bandini, Luís Eduardo S. Vitolano, Jacqueline J. C. Nobre, Cristiane T. Moreira, and et al. 2023. "Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils" Coatings 13, no. 5: 847. https://doi.org/10.3390/coatings13050847
APA StyleOliveira Filho, J. G. d., Duarte, L. G. R., Silva, Y. B. B., Milan, E. P., Santos, H. V., Moura, T. C., Bandini, V. P., Vitolano, L. E. S., Nobre, J. J. C., Moreira, C. T., Mitsuyuki, M. C., Bogusz Junior, S., & Ferreira, M. D. (2023). Novel Approach for Improving Papaya Fruit Storage with Carnauba Wax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils. Coatings, 13(5), 847. https://doi.org/10.3390/coatings13050847