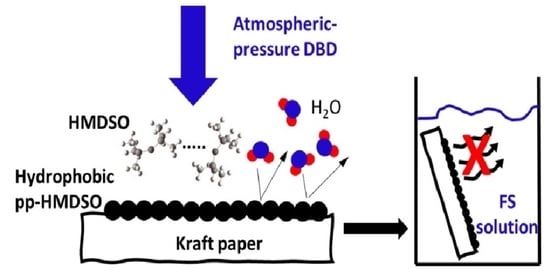
Plasma-Deposited Organosilicon Hydrophobic Coatings on Cellulosic Materials for Wet Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Deposition Conditions
2.2. Immersion Conditions
2.3. Characterization of Plasma-Deposited Coatings
2.4. Statistical Analysis
3. Results and Discussion
3.1. Wettability and Absorption
3.1.1. Effect of Time
3.1.2. Effects of Stirring Rate and Temperature
3.2. Chemical Composition
3.3. Morphology
3.4. Release of Silicon
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciolacu, D.; Popa, V.I. Cellulose allomorphs: Structure, accessibility and reactivity. Environ. Eng. Manag. J. 2011, 10, 467–468. [Google Scholar]
- Gadhave, R.V.; Gadhave, C.R.; Dhawale, P.V. Plastic-free bioactive paper coatings, way to next-generation sustainable paper packaging application: A review. Green Sustain. Chem. 2022, 12, 9–27. [Google Scholar] [CrossRef]
- Dey, A.; Sengupta, P.; Pramanik, N.K.; Alam, T. Paper and other pulp based eco-friendly moulded materials for food packaging applications: A review. J. Postharvest Technol. 2020, 8, 01–21. [Google Scholar]
- Wang, Q.; Sun, J.; Yao, Q.; Ji, C.; Liu, J.; Zhu, Q. 3D printing with cellulose materials. Cellulose 2018, 25, 4275–4301. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Yang, Y.; Chu, J.; He, B. Emerging paper microfluidic devices. Analyst 2019, 144, 6497–6511. [Google Scholar] [CrossRef] [PubMed]
- Andresen, M.; Johansson, L.-S.; Tanem, B.S.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar] [CrossRef]
- Garcia-Ubasart, J.; Colom, J.F.; Vila, C.; Gomez Hernandez, N.; Blanca Roncero, M.; Vidal, T. A new procedure for the hydrophobization of cellulose fibre using laccase and a hydrophobic phenolic compound. Bioresour. Technol. 2012, 112, 341–344. [Google Scholar] [CrossRef]
- Forsman, N.; Lozhechnikova, A.; Khakalo, A.; Johansson, L.S.; Vartiainen, J.; Osterberg, M. Layer-by-layer assembled hydrophobic coatings for cellulose nanofibril films and textiles, made of polylysine and natural wax particles. Carbohydr. Polym. 2017, 173, 392–402. [Google Scholar] [CrossRef]
- Levasseur, O.; Vlad, M.; Profili, J.; Gherardi, N.; Sarkissian, A.; Stafford, L. Deposition of fluorocarbon groups on wood surfaces using the jet of an atmospheric-pressure dielectric barrier discharge. Wood Sci. Technol. 2017, 51, 1339–1352. [Google Scholar] [CrossRef]
- Rodionova, G.; Lenes, M.; Eriksen, Ø.; Gregersen, Ø. Surface chemical modification of microfibrillated cellulose: Improvement of barrier properties for packaging applications. Cellulose 2010, 18, 127–134. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Zhao, Z.; Xu, S. Process variables and the performance of soybean-oil rosin-based polyester as an internal sizing agent. BioResources 2019, 14, 9183–9197. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.; Trindade, T.; Neto, C.P.; Gandini, A. Superhydrophobic cellulose nanocomposites. J. Colloid Interface Sci. 2008, 324, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Guthrie, J.T.; Perrier, S. graft polymerization: Grafting poly(styrene) from cellulose via reversible addition-fragmentation chain transfer (RAFT) polymerization. Macromolecules 2005, 38, 10363–10372. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Borsa, J.; Rácz, I. Hydrophilic/hydrophobic character of grafted cellulose. Radiat. Phys. Chem. 2010, 79, 467–470. [Google Scholar] [CrossRef]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through surface-initiated atom transfer radical polymerization (SI-ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef]
- Avramidis, G.; Hauswald, E.; Lyapin, A.; Militz, H.; Viöl, W.; Wolkenhauer, A. Plasma treatment of wood and wood-based materials to generate hydrophilic or hydrophobic surface characteristics. Wood Mater. Sci. Eng. 2009, 4, 52–60. [Google Scholar] [CrossRef]
- Bente, M.; Avramidis, G.; Förster, S.; Rohwer, E.G.; Viöl, W. Wood surface modification in dielectric barrier discharges at atmospheric pressure for creating water repellent characteristics. Holz Als Roh Werkst. 2004, 62, 157–163. [Google Scholar] [CrossRef]
- Blanchard, N.E.; Naik, V.V.; Geue, T.; Kahle, O.; Hegemann, D.; Heuberger, M. Response of plasma-polymerized hexamethyldisiloxane films to aqueous environments. Langmuir 2015, 31, 12944–12953. [Google Scholar] [CrossRef]
- Cunha, A.G.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Orblin, E.; Fardim, P. Highly hydrophobic biopolymers prepared by the surface pentafluorobenzoylation of cellulose substrates. Biomacromolecules 2007, 8, 1347–1352. [Google Scholar] [CrossRef]
- Deslandes, Y.; Pleizier, G.; Poire, E.; Sapieha, S.; Wertheimer, M.R.; Sacher, E. The surface modification of pure cellulose paper induced by low-pressure nitrogen plasma treatment. Plasmas Polym. 1998, 3, 61–76. [Google Scholar] [CrossRef]
- Foroughi Mobarakeh, L.; Jafari, R.; Farzaneh, M. Superhydrophobic Surface Elaboration Using Plasma Polymerization of Hexamethyldisiloxane (HMDSO). Adv. Mater. Res. 2011, 409, 783–787. [Google Scholar] [CrossRef]
- Levasseur, O.; Stafford, L.; Gherardi, N.; Naudé, N.; Blanchard, V.; Blanchet, P.; Riedl, B.; Sarkissian, A. Deposition of hydrophobic functional groups on wood surfaces using atmospheric-pressure dielectric barrier discharge in helium-hexamethyldisiloxane gas mixtures. Plasma Process. Polym. 2012, 9, 1168–1175. [Google Scholar] [CrossRef]
- Nithya, E.; Radhai, R.; Rajendran, R.; Shalini, S.; Rajendran, V.; Jayakumar, S. Synergetic effect of DC air plasma and cellulase enzyme treatment on the hydrophilicity of cotton fabric. Carbohydr. Polym. 2011, 83, 1652–1658. [Google Scholar] [CrossRef]
- Nouicer, I.; Sahli, S.; Kihel, M.; Ziari, Z.; Bellel, A.; Raynaud, P. Superhydrophobic surface produced on polyimide and silicon by plasma enhanced chemical vapour deposition from hexamethyldisiloxane precursor. Int. J. Nanotechnol. 2015, 12, 597–607. [Google Scholar] [CrossRef]
- Odrášková, M.; Szalay, Z.; Ráheľ, J.; Zahoranová, A.; Černák, M. Diffuse coplanar surface barrier discharge assisted deposition of water repellent films from N2/HMDSO mixtures on wood surface. In Proceedings of the 28th ICPIG, Prague, Czech Republic, 15–20 July 2007. [Google Scholar]
- Enache, I.; Caquineau, H.; Gherardi, N.; Paulmier, T.; Maechler, L.; Massines, F. Transport phenomena in an atmospheric-pressure Townsend discharge fed by N2/N2O/HMDSO mixtures. Plasma Process. Polym. 2007, 4, 806–814. [Google Scholar] [CrossRef]
- Levasseur, O.; Stafford, L.; Gherardi, N.; Naudé, N.; Beche, E.; Esvan, J.; Blanchet, P.; Riedl, B.; Sarkissian, A. Role of substrate outgassing on the formation dynamics of either hydrophilic or hydrophobic wood surfaces in atmospheric-pressure, organosilicon plasmas. Surf. Coat. Technol. 2013, 234, 42–47. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Chang, B.-S.; Lin, J.-Y. Improvement of water and oil repellency on wood substrates by using fluorinated silica nanocoating. Appl. Surf. Sci. 2011, 257, 7997–8002. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of wood with silicon compounds. Treatment systems based on organic silicon compounds–a review. Wood Sci. Technol. 2004, 37, 453–461. [Google Scholar] [CrossRef]
- Podgorski, L.; Bousta, C.; Schambourg, F.; Maguin, J.; Chevet, B. Surface modification of wood by plasma polymerisation. Pigment Resin Technol. 2001, 31, 33–40. [Google Scholar] [CrossRef]
- Potočňáková, L.; Hnilica, J.; Kudrle, V. Increase of wettability of soft- and hardwoods using microwave plasma. Int. J. Adhes. Adhes. 2013, 45, 125–131. [Google Scholar] [CrossRef]
- Riedl, B.; Angel, C.; Prégent, J.; Blanchet, P.; Stafford, L. Effect of wood surface modification by atmospheric-pressure plasma on waterborne coating adhesion. BioResources 2014, 9, 4908–4923. [Google Scholar] [CrossRef]
- Sèbe, G.; Brook, M.A. Hydrophobization of wood surfaces: Covalent grafting of silicone polymers. Wood Sci. Technol. 2001, 35, 269–282. [Google Scholar] [CrossRef]
- Rehn, P.; Wolkenhauer, A.; Bente, M.; Förster, S.; Viöl, W. Wood surface modification in dielectric barrier discharges at atmospheric pressure. Surf. Coat. Technol. 2003, 174–175, 515–518. [Google Scholar] [CrossRef]
- Vander Wielen, L.C.; Östenson, M.; Gatenholm, P.; Ragauskas, A.J. Surface modification of cellulosic fibers using dielectric-barrier discharge. Carbohydr. Polym. 2006, 65, 179–184. [Google Scholar] [CrossRef]
- Lau, O.; Wong, S. Contamination in food from packaging material. J. Chromatogr. A 2000, 882, 255–270. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- Bhunia, K.; Sablani, S.S.; Tang, J.; Rasco, B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage. Compr. Rev. Food Sci. Food Saf. 2013, 12, 523–545. [Google Scholar] [CrossRef]
- Reid, R.C.; Sldman, K.R.; Schwope, A.D.; Till, D.E. Loss of adjuvants from polymer films to foods or food simulants. effect of the external phase. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 580–587. [Google Scholar] [CrossRef]
- Kathuria, A.; Zhang, S. Sustainable and repulpable barrier coatings for fiber-based materials for food packaging: A review. Front. Mater. 2022, 9, 110556. [Google Scholar] [CrossRef]
- Al Salloum, H.; Saunier, J.; Tfayli, A.; Yagoubi, N. Studying DEHP migration in plasticized PVC used for blood bags by coupling Raman confocal microscopy to UV spectroscopy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 56–62. [Google Scholar] [CrossRef]
- Fankhauser-Noti, A.; Biedermann-Brem, S.; Grob, K. PVC plasticizers/additives migrating from the gaskets of metal closures into oily food: Swiss market survey June 2005. Eur. Food Res. Technol. 2006, 223, 447–453. [Google Scholar] [CrossRef]
- Kawamura, Y.; Ogawa, Y.; Mutsuga, M. Migration of nonylphenol and plasticizers from polyvinyl chloride stretch film into food simulants, rapeseed oil, and foods. Food Sci. Nutr. 2017, 5, 390–398. [Google Scholar] [CrossRef]
- Sero, R.; Nunez, O.; Javier Santos, F.; Moyano, E. Analytical methods for the determination of plasticizers in food and beverages. Curr. Anal. Chem. 2018, 14, 306–324. [Google Scholar] [CrossRef]
- Zygoura, P.D.; Goulas, A.E.; Riganakos, K.A.; Kontominas, M.G. Migration of di-(2-ethylhexyl)adipate and acetyltributyl citrate plasticizers from food-grade PVC film into isooctane: Effect of gamma radiation. J. Food Eng. 2007, 78, 870–877. [Google Scholar] [CrossRef]
- de Freitas, A.S.M.; Maciel, C.C.; Rodrigues, J.S.; Ribeiro, R.P.; Delgado-Silva, A.O.; Rangel, E.C. Organosilicon films deposited in low-pressure plasma from hexamethyldisiloxane—A review. Vacuum 2021, 194. [Google Scholar] [CrossRef]
- Coclite, A.M.; Gleason, K.K. Initiated PECVD of organosilicon coatings: A new strategy to enhance monomer structure retention. Plasma Processes Polym. 2012, 9, 425–434. [Google Scholar] [CrossRef]
- Salamianski, A.E.; Agabekov, V.E.; Chishankov, I.G.; Matveenko, Y.V.; Melnikova, G.B.; Nguyen, T.D.; Vu, K.O.; Pham, G.V.; Trinh, A.T.; Tran, D.L.; et al. Tribological and anticorrosion behavior of coating systems based on polyurethane and organic silicon compounds. Prot. Met. Phys. Chem. Surf. 2021, 57, 1034–1039. [Google Scholar] [CrossRef]
- Taggart, O. A Study of the Mechanical Properties of Silicon-based Thin Films Deposited by ECR-PECVD and ICP-CVD. PhD Thesis, McMaster University, Hamilton, Ontario, 2013. [Google Scholar]
- Angelini, E.; d’Agostino, R.; Fracassi, F.; Grassini, S.; Rosalbino, F. Surface analysis of PECVD organosilicon films for corrosion protection of steel substrates. Surf. Interface Anal. 2002, 34, 155–159. [Google Scholar] [CrossRef]
- Carpentier, J.; Grundmeier, G. Chemical structure and morphology of thin bilayer and composite organosilicon and fluorocarbon microwave plasma polymer films. Surf. Coat. Technol. 2005, 192, 189–198. [Google Scholar] [CrossRef]
- Babaei, S.; Profili, J.; Al Rashidi, M.; Dorris, A.; Beck, S.; Asadollahi, S.; Sarkassian, A.; Stafford, L. Permeation properties of a plasma-processed organosilicon-carboxymethylcellulose bilayer on fibrillated cellulosic films for sustainable packaging applications. Cellulose 2023. submitted. [Google Scholar]
- FDA. Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations); Food and Drug Administration: Silver Spring, MD, USA, 2007. [Google Scholar]
- Babaei, S.; Profili, J.; Asadollahi, S.; Sarkassian, A.; Dorris, A.; Beck, S.; Stafford, L. Analysis of transport phenomena during plasma deposition of hydrophobic coatings on porous cellulosic substrates in plane-to-plane dielectric barrier discharges at atmospheric pressure. Plasma Process. Polym. 2020, 17, 105865. [Google Scholar] [CrossRef]
- Profili, J.; Levasseur, O.; Koronai, A.; Stafford, L.; Gherardi, N. Deposition of nanocomposite coatings on wood using cold discharges at atmospheric pressure. Surf. Coat. Technol. 2017, 309, 729–737. [Google Scholar] [CrossRef]
- Profili, J.; Asadollahi, S.; Vinchon, P.; Dorris, A.; Beck, S.; Sarkassian, A.; Stafford, L. Recent progress on organosilicon coatings deposited on bleached unrefined Kraft paper by non-thermal plasma process at atmospheric pressure. Prog. Org. Coat. 2020, 147, 105865. [Google Scholar] [CrossRef]
- HPFB. Information Requirements for Food Packaging Submissions. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/guidance-documents/information-requirements-food-packaging-submissions.html (accessed on 7 February 2023).
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of mechanical properties, migration, and potential applications in active food packaging systems containing nanoclays and nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Brown, F.; Diller, K.R. Calculating the optimum temperature for serving hot beverages. Burns 2008, 34, 648–654. [Google Scholar] [CrossRef]
- OriginPro, Version 2015; OriginLab Corporation: Northampton, MA, USA, 2015.
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma treatment of polymers to generate stable, hydrophobic surfaces. Plasmas Polym. 2001, 6, 221–235. [Google Scholar] [CrossRef]
- Rahmati, S. Direct Rapid Tooling. In Comprehensive Materials Processing; Hashmi, S., Ed.; Elsevier: Amsterdam, Netherlands, 2014. [Google Scholar]
- Busnel, F.; Blanchard, V.; Prégent, J.; Stafford, L.; Riedl, B.; Blanchet, P.; Sarkissian, A. Modification of sugar maple (acer saccharum) and black spruce (picea mariana) wood surfaces in a dielectric barrier discharge (DBD) at atmospheric pressure. J. Adhes. Sci. Technol. 2010, 24, 1401–1413. [Google Scholar] [CrossRef]
- Stuart, S. Organosilicon Chemistry: Special Lectures Presented at the International Symposium on Organosilicon Chemistry; Elsevier: Amsterdam, Netherlands, 1966. [Google Scholar] [CrossRef]
- Durbin, R.P. Osmotic flow of water across permeable cellulose membranes. J. Gen. Physiol. 1960, 44, 315–326. [Google Scholar] [CrossRef]
- Mahomed, A.; Hukins, D.W.; Kukureka, S.N. Swelling of medical grade silicones in liquids and calculation of their cross-link densities. Med. Eng. Phys. 2010, 32, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, S.; Profili, J.; Farzaneh, M.; Stafford, L. Development of organosilicon-based superhydrophobic coatings through atmospheric pressure plasma polymerization of HMDSO in nitrogen plasma. Materials 2019, 12, 219. [Google Scholar] [CrossRef]
- Pulpytel, J.; Kumar, V.; Peng, P.; Micheli, V.; Laidani, N.; Arefi-Khonsari, F. Deposition of organosilicon coatings by a non-equilibrium atmospheric pressure plasma jet: Design, analysis and macroscopic scaling law of the process. Plasma Process. Polym. 2011, 8, 664–675. [Google Scholar] [CrossRef]
- Denton, P. 47—The water absorption characteristics of monofilament cellulose. J. Text. Inst. Trans. 1956, 47, T570–T585. [Google Scholar] [CrossRef]
- Minelli, M.; Baschetti, M.G.; Doghieri, F.; Ankerfors, M.; Lindström, T.; Siró, I.; Plackett, D. Investigation of mass transport properties of microfibrillated cellulose (MFC) films. J. Membr. Sci. 2010, 358, 67–75. [Google Scholar] [CrossRef]
- Kirk, C.T. Quantitative analysis of the effect of disorder-induced mode coupling on infrared absorption in silica. Phys. Rev. B Condens. Matter 1988, 38, 1255–1273. [Google Scholar] [CrossRef]
- Duncan, B.; Urquhart, J.; Roberts, S. Review of Measurement and Modelling of Permeation and Diffusion in Polymers; National Physical Laboratory: Teddington, UK, 2005. [Google Scholar]
- Russell, S.P.; Weinkauf, D.H. Vapor sorption in plasma polymerized vinyl acetate and methyl methacrylate thin films. Polymer 2001, 42, 2827–2836. [Google Scholar] [CrossRef]
- Tsige, M.; Grest, G.S. Interdiffusion of solvent into glassy polymer films: A molecular dynamics study. J. Chem. Phys. 2004, 121, 7513–7519. [Google Scholar] [CrossRef] [PubMed]
- Doremus, R.H. Diffusion of water in silica glass. J. Mater. Res. 1995, 10, 2379–2389. [Google Scholar] [CrossRef]
- Erlat, A.G.; Spontak, R.J. SiOx gas barrier coatings on polymer substrates: Morphology and gas transport considerations. J. Phys. Chem. B 1999, 103, 6047–6055. [Google Scholar] [CrossRef]
- Li, K.; Meichsner, J. Gas-separating properties of membranes coated by HMDSO plasma polymer. Surf. Coat. Technol. 1999, 116–119, 841–847. [Google Scholar] [CrossRef]
- Palaskar, S.; Kale, K.H.; Nadiger, G.S.; Desai, A.N. Dielectric barrier discharge plasma induced surface modification of polyester/cotton blended fabrics to impart water repellency using HMDSO. J. Appl. Polym. Sci. 2011, 122, 1092–1100. [Google Scholar] [CrossRef]
- FDA. Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients; Food and Drug Admistration: Silver Spring, MD, USA, 2005. [Google Scholar]
- ASTM D859-16(2021)e1; Standard Test Method for Silica in Water. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Baner, A.; Brandsch, J.; Franz, R.; Piringer, O. The application of a predictive migration model for evaluating the compliance of plastic materials with European food regulations. Food Addit. Contam. 1996, 13, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, F.; Hu, Y.; Kong, Q.; Liu, C.; Rahman, M.A.; Zhou, Y.; Jia, P. Highly branched and nontoxic plasticizers based on natural cashew shell oil by a facile and sustainable way. J. Clean. Prod. 2020, 252, 119597. [Google Scholar] [CrossRef]
- Reynier, A.; Dole, P.; Feigenbaum, A. Prediction of worst case migration: Presentation of a rigorous methodology. Food Addit. Contam. 1999, 16, 137–152. [Google Scholar] [CrossRef] [PubMed]








| Variable | Time (h) | Temperature (°C) | RPM * (min−1) |
|---|---|---|---|
| Parametric study 1 | 0.5 3 16 24 | Constant (40) | Constant (50) |
| Parametric study 2 | Constant (3) | 23 40 65 | Constant (50) |
| Parametric study 3 | Constant (3) | Constant (65) | 50 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Profili, J.; Babaei, S.; Al Rashidi, M.; Dorris, A.; Asadollahi, S.; Sarkissian, A.; Stafford, L. Plasma-Deposited Organosilicon Hydrophobic Coatings on Cellulosic Materials for Wet Packaging Applications. Coatings 2023, 13, 924. https://doi.org/10.3390/coatings13050924
Profili J, Babaei S, Al Rashidi M, Dorris A, Asadollahi S, Sarkissian A, Stafford L. Plasma-Deposited Organosilicon Hydrophobic Coatings on Cellulosic Materials for Wet Packaging Applications. Coatings. 2023; 13(5):924. https://doi.org/10.3390/coatings13050924
Chicago/Turabian StyleProfili, Jacopo, Sara Babaei, Mariam Al Rashidi, Annie Dorris, Siavash Asadollahi, Andranik Sarkissian, and Luc Stafford. 2023. "Plasma-Deposited Organosilicon Hydrophobic Coatings on Cellulosic Materials for Wet Packaging Applications" Coatings 13, no. 5: 924. https://doi.org/10.3390/coatings13050924
APA StyleProfili, J., Babaei, S., Al Rashidi, M., Dorris, A., Asadollahi, S., Sarkissian, A., & Stafford, L. (2023). Plasma-Deposited Organosilicon Hydrophobic Coatings on Cellulosic Materials for Wet Packaging Applications. Coatings, 13(5), 924. https://doi.org/10.3390/coatings13050924