Surfaces Bearing Fluorinated Nucleoperfluorolipids for Potential Anti-Graffiti Surface Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. General Procedure for Electropolymerization
2.3. General Procedure for PEDOT Surface Modification
2.4. Surface Characterization
3. Results
3.1. Synthesis
3.2. Electrodeposition
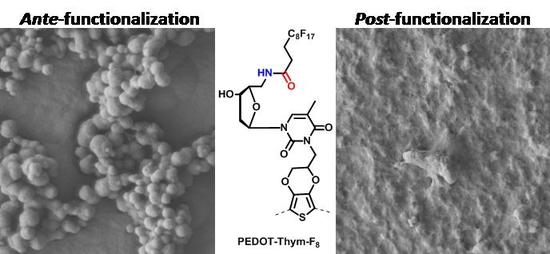
3.3. PEDOT-Thym-N3 Post-Functionalization
3.4. Surface Roughness
3.5. Surface Wettability
3.6. Surface Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Darmanin, T.; Guittard, F. Recent advances in the potential applications of bioinspired superhydrophobic materials. J. Mater. Chem. A 2014, 2, 16319–16359. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, Y.; Zhai, J.; Jiang, L. Bioinspired super-antiwetting interfaces with special liquid–Solid adhesion. Acc. Chem. Res. 2010, 43, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef] [PubMed]
- Rabea, A.M.; Mohseni, M.; Mirabedini, S.M.; Tabatabaei, M.H. Surface analysis and anti-graffiti behavior of a weathered polyurethane-based coating embedded with hydrophobic nano silica. Appl. Surf. Sci. 2012, 258, 4391–4396. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M. Surface characterization and effectiveness evaluation of anti-graffiti coatings on highly porous stone materials. Appl. Surf. Sci. 2014, 288, 466–477. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Sun, Z.; Liao, T.; Liu, K.; Jiang, L.; Kim, J.H.; Dou, S.X. Fly-eye inspired superhydrophobic anti-fogging inorganic nanostructures. Small 2014, 10, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Hensel, R.; Neinhuis, C.; Werner, C. The springtail cuticle as a blueprint for omniphobic surfaces. Chem. Soc. Rev. 2016, 45, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Hensel, R.; Neinhuis, C.; Werner, C. Wetting resistance at its topographical limit: The benefit of mushroom and serif T structures. Langmuir 2013, 29, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Bormashenko, E. Physics of solid–liquid interfaces: From the Young equation to the superhydrophobicity. Low Temp. Phys. 2016, 42, 622–635. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Marmur, A. From hygrophilic to superhygrophobic: Theoretical conditions for making high-contact-angle surfaces from low-contact-angle materials. Langmuir 2008, 24, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Zha, J.; Batisse, N.; Claves, D.; Dubois, M.; Frezet, L.; Kharitonov, A.P.; Alekseiko, L.N. Superhydrophocity via gas-phase monomers grafting onto carbon nanotubes. Prog. Surf. Sci. 2016, 91, 57–71. [Google Scholar] [CrossRef]
- Chu, Z.; Olveira, S.; Seeger, S. A facile, sustainable strategy towards the preparation of silicone nanofilaments and their use as antiwetting coatings. ChemistrySelect 2017, 2, 5463–5468. [Google Scholar] [CrossRef]
- Mortier, C.; Darmanin, T.; Guittard, F. Direct electrodeposition of superhydrophobic and highly oleophobic poly(3,4-ethylenedioxypyrrole (PEDOP) and poly(3,4-propylenedioxypyrrole) (PProDOP) nanofibers. ChemNanoMat 2017. [Google Scholar] [CrossRef]
- Godeau, G.; Darmanin, T.; Guittard, F. Nucleoside surface as platform for the control of surface hydrophobicity. RSC Adv. 2016, 6, 62471–62477. [Google Scholar] [CrossRef]
- Godeau, G.; Barthélémy, P. Glycosyl-nucleoside lipids as low-molecular-weight gelators. Langmuir 2009, 25, 8447–8450. [Google Scholar] [CrossRef] [PubMed]
- Dolain, C.; Patwa, A.; Godeau, G.; Barthélémy, P. Nucleic acid based fluorinated derivatives: New tools for biomedical applications. Appl. Sci. 2012, 2, 245–259. [Google Scholar] [CrossRef]
- Burés, J.; Martín, M.; Urpí, F.; Vilarrasa, J. Catalytic Staudinger-Vilarrasa reaction for the direct ligation of carboxylic acids and azides. J. Org. Chem. 2009, 74, 2203–2206. [Google Scholar] [CrossRef] [PubMed]
- Godeau, G.; Darmanin, T.; Guittard, F. Ante versus post-functionalization to control surface structures with superhydrophobic and superoleophobic properties. RSC Adv. 2015, 5, 63945–63951. [Google Scholar] [CrossRef]
- Godeau, G.; Staedel, C.; Barthélémy, P. Lipid-conjugated oligonucleotides via “click chemistry” efficiently inhibit hepatitis C virus translation. J. Med. Chem. 2008, 51, 4374–4376. [Google Scholar] [CrossRef] [PubMed]












| Momomer | Oxydation Potention (V) | Depostion Range (V) |
|---|---|---|
| EDOT-T-N3 | 1.49 | −1 to 1.45 |
| EDOT-T-F4 | 1.44 | −1 to 1.38 |
| EDOT-T-F6 | 1.48 | −1 to 1.46 |
| EDOT-T-F8 | 1.47 | −1 to 1.43 |
| Water | PEDOT-T-N3 (°) | PEDOT-T-F4 (°) | PEDOT-T-F6 (°) | PEDOT-T-F8 (°) | |
| Ante | 1 scan | 73 ± 0.5 | 103 ±1 | 118 ± 1 | 115 ± 3 |
| 3 scans | 72 ± 2 | 113 ± 1 | 131 ± 1 | 125 ± 1 | |
| 5 scans | 77 ± 2 | 123 ± 1.5 | 117 ± 1 | 137 ± 1 | |
| Post | 3 scans | 72 ± 2 | 106.5 ± 2 | 117 ± 1 | 112 ± 3 |
| CH2I2 | – | PEDOT-T-F4 (°) | PEDOT-T-F6 (°) | PEDOT-T-F8 (°) | |
| Ante | 1 scan | – | 81 ± 4 | 98 ± 2 | 95 ± 4 |
| 3 scans | – | 92 ± 2 | 110 ± 1 | 102 ± 3 | |
| 5 scans | – | 104 ± 2 | 91 ± 6 | 118 ± 1 | |
| Post | 3 scans | – | 84 ± 5 | 97 ± 3 | 83 ± 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godeau, G.; Guittard, F.; Darmanin, T. Surfaces Bearing Fluorinated Nucleoperfluorolipids for Potential Anti-Graffiti Surface Properties. Coatings 2017, 7, 220. https://doi.org/10.3390/coatings7120220
Godeau G, Guittard F, Darmanin T. Surfaces Bearing Fluorinated Nucleoperfluorolipids for Potential Anti-Graffiti Surface Properties. Coatings. 2017; 7(12):220. https://doi.org/10.3390/coatings7120220
Chicago/Turabian StyleGodeau, Guilhem, Frédéric Guittard, and Thierry Darmanin. 2017. "Surfaces Bearing Fluorinated Nucleoperfluorolipids for Potential Anti-Graffiti Surface Properties" Coatings 7, no. 12: 220. https://doi.org/10.3390/coatings7120220
APA StyleGodeau, G., Guittard, F., & Darmanin, T. (2017). Surfaces Bearing Fluorinated Nucleoperfluorolipids for Potential Anti-Graffiti Surface Properties. Coatings, 7(12), 220. https://doi.org/10.3390/coatings7120220