Tuning Nucleation Sites to Enable Monolayer Perovskite Films for Highly Efficient Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursor and Perovskite Film Preparation
2.2. Perovskite Solar Cell Fabrication
2.3. Characterization
3. Results and Discussion
3.1. Nucleation Behavior of the Perovskite Precursor under Natural Drying Conditions
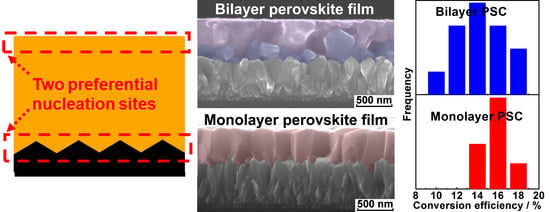
3.2. Correlation between Nucleation Sites and Microstructures of Perovskite Films
3.3. Photovoltaic Performance of the Perovskite Solar Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Il Seok, S.; Lee, J.; Seo, J. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 2018, 3, 682–689. [Google Scholar] [CrossRef]
- Singh, T.; Miyasaka, T. Stabilizing the efficiency beyond 20% with a mixed cation perovskite solar cell fabricated in ambient air under controlled humidity. Adv. Energy Mater. 2018, 8, 1700677. [Google Scholar] [CrossRef]
- Niu, G.D.; Guo, X.D.; Wang, L.D. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Ye, J.; Liu, G.; Jiang, L.; Zheng, H.; Zhu, L.; Zhang, X.; Wang, H.; Pan, X.; Dai, S. Crack-free perovskite layers for high performance and reproducible devices via improved control of ambient conditions during fabrication. Appl. Surf. Sci. 2017, 407, 427–433. [Google Scholar] [CrossRef]
- Qiu, W.; Merckx, T.; Jaysankar, M.; de la Huerta, C.M.; Rakocevic, L.; Zhang, W.; Paetzold, U.W.; Gehlhaar, R.; Froyen, L.; Poortmans, J.; et al. Pinhole-free perovskite films for efficient solar modules. Energy Environ. Sci. 2016, 9, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Li, M.; Shi, X.B.; Ma, H.; Wang, Z.K.; Liao, L.S. Controllable perovskite crystallization by water additive for high-performance solar cells. Adv. Funct. Mater. 2015, 25, 6671–6678. [Google Scholar] [CrossRef]
- Das, S.; Yang, B.; Gu, G.; Joshi, P.C.; Ivanov, I.N.; Rouleau, C.M.; Aytug, T.; Geohegan, D.B.; Xiao, K. High-Performance Flexible Perovskite Solar Cells by Using a Combination of Ultrasonic Spray-Coating and Low Thermal Budget Photonic Curing. ACS Photonics 2015, 2, 680–686. [Google Scholar] [CrossRef]
- Xu, H.T.; Wu, Y.L.; Xu, F.Z.; Zhu, J.B.; Ni, C.W.; Wang, W.Z.; Hong, F.; Xu, R.; Xu, F.; Huang, J.; et al. Grain growth study of perovskite thin films prepared by flash evaporation and its effect on solar cell performance. RSC Adv. 2016, 6, 48851–48857. [Google Scholar] [CrossRef]
- Du, T.; Wang, N.; Chen, H.J.; Lin, H.; He, H.C. Comparative study of vapor- and solution-crystallized perovskite for planar heterojunction solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3382–3388. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zheng, J.; Zheng, L.; Yan, X.; Lin, H.; Zhang, F. Crack-free CH3NH3PbI3 layer via continuous dripping method for high-performance mesoporous perovskite solar cells. Appl. Surf. Sci. 2017, 392, 960–965. [Google Scholar] [CrossRef]
- Wang, F.; Yu, H.; Xu, H.H.; Zhao, N. A newprecursor compound for highly efficient solution-processed perovskite solar cells. Adv. Funct. Mater. 2015, 25, 1120–1126. [Google Scholar] [CrossRef]
- Li, C.; Guo, Q.; Qiao, W.Y.; Chen, Q.; Ma, S.; Pan, X.; Wang, F.Z.; Yao, J.X.; Zhang, C.F.; Xiao, M.; et al. Efficient lead acetate sourced planar heterojunction perovskite solar cells with enhanced substrate coverage via one-step spin-coating. Org. Electron. 2016, 33, 194–200. [Google Scholar] [CrossRef]
- Park, B.-S.; Lee, S.; Yoon, S.; Ha, T.-J.; Kang, D.-W. Methylammonium lead mixed halide films processed with a new composition for planar perovskite solar cells. Appl. Surf. Sci. 2018, 427, 421–426. [Google Scholar] [CrossRef]
- Yan, J.; Ke, X.; Chen, Y.; Zhang, A.; Zhang, B. Effect of modulating the molar ratio of organic to inorganic content on morphology, optical absorption and photoluminescence of perovskite CH3NH3PbBr3 films. Appl. Surf. Sci. 2015, 351, 1191–1196. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Wu, L.L.; Li, W.; Chen, F.; Xiao, B.Q.; Zhang, J.Q.; Feng, L.H. Highly reproducible perovskite solar cells with excellent CH3NH3PbI3-xClx film morphology fabricated via high precursor concentration. RSC Adv. 2016, 6, 51279–51285. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, F.; Chen, L.; Li, J. Adsorption of molecular additive onto lead halide perovskite surfaces: A computational study on Lewis base thiophene additive passivation. Appl. Surf. Sci. 2018, 443, 176–183. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.W.; Sun, P.; Ma, W.L.; Chen, Z.K. Additive to regulate the perovskite crystal film growth in planar heterojunction solar cells. Appl. Phys. Lett. 2015, 106, 033901. [Google Scholar] [CrossRef]
- Ding, Y.L.; Yao, X.; Zhang, X.D.; Wei, C.C.; Zhao, Y. Surfactant enhanced surface coverage of CH3NH3PbI3-xClx perovskite for highly efficient mesoscopic solar cells. J. Power Sources 2014, 272, 351–355. [Google Scholar] [CrossRef]
- Bi, C.; Wang, Q.; Shao, Y.C.; Yuan, Y.B.; Xiao, Z.G.; Huang, J.S. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cojocaru, L.; Uchida, S.; Sanehira, Y.; Nakazaki, J.; Kubo, T.; Segawa, H. Surface treatment of the compact TiO2 layer for efficient planar heterojunction perovskite solar cells. Chem. Lett. 2015, 44, 674–676. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Yuan, D.X.; Mu, H.R.; Femi, I.; Bao, Q.; Liao, L.S. Efficiency enhancement of perovskite solar cells by pumping away the solvent of precursor film before annealing. Nanoscale Res. Lett. 2016, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Z.; Dkhissi, Y.; Huang, W.C.; Xiao, M.D.; Benesperi, I.; Rubanov, S.; Zhu, Y.; Lin, X.F.; Jiang, L.C.; Zhou, Y.C.; et al. Gas-assisted preparation of lead iodide perovskite films consisting of a monolayer of single crystalline grains for high efficiency planar solar cells. Nano Energy 2014, 10, 10–18. [Google Scholar] [CrossRef]
- Kim, J.H.; Williams, S.T.; Cho, N.; Chueh, C.-C.; Jen, A.K.Y. Enhanced environmental stability of planar heterojunction perovskite solar cells based on blade-coating. Adv. Energy Mater. 2015, 5, 1401229. [Google Scholar] [CrossRef]
- Lewis, A.E.; Zhang, Y.; Gao, P.; Nazeeruddin, M.K. Unveiling the concentration-dependent grain growth of perovskite films from one- and two-step deposition methods: implications for photovoltaic application. ACS Appl. Mater. Interfaces 2017, 9, 25063–25066. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.-K.; Ding, X.-H.; Wu, Y.-H.; Zhu, J.; Hayat, T.; Alsaedi, A.; Xu, Y.-F.; Li, Z.-Q.; Yang, S.-F.; Dai, S.-Y. Temperature-assisted rapid nucleation: a facile method to optimize the film morphology for perovskite solar cells. J. Mater. Chem. A 2017, 5, 20327–20333. [Google Scholar] [CrossRef]
- Ding, B.; Li, Y.; Huang, S.-Y.; Chu, Q.-Q.; Li, C.-X; Li, C.-J.; Yang, G.-J. Material nucleation/growth competition tuning towards highly reproducible planar perovskite solar cells with efficiency exceeding 20%. J. Mater. Chem. A 2017, 5, 6840–6848. [Google Scholar]
- Li, Y.; He, X.L.; Ding, B.; Gao, L.L.; Yang, G.J.; Li, C.X.; Li, C.J. Realizing full coverage of perovskite film on substrate surface during solution processing: Characterization and elimination of uncovered surface. J. Power Sources 2016, 320, 204–211. [Google Scholar] [CrossRef]
- Arefpour, A.; Shams Soolari, L.; Monshi, A. Improving mold powder through crystallization using calcium fluoride and manganese oxide for continuous casting of steel. J. Adv. Ceram. 2014, 3, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Gautam, C.R.; Madheshiya, A.; Mazumder, R. Preparation, crystallization, microstructure and dielectric properties of lead bismuth titanate borosilicate glass ceramics. J. Adv. Ceram. 2014, 3, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ba, G.; Qi, X.; Li, X.; Song, Y.; Li, B. Nanocrystalline BaTi2O5 dielectric ceramic prepared by full crystallization from containerless solidified glass. J. Adv. Ceram. 2016, 5, 77–83. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, D.; Gu, H.; Xing, J.; Xiong, P.; Wan, D.; Gao, Y. Crystallization and inter-diffusional behaviors in the formation of VO2(B) thin film with layered W-doping. J. Adv. Ceram. 2017, 6, 196–206. [Google Scholar] [CrossRef]
- Li, Y.; Ding, B.; Chu, Q.Q.; Yang, G.J.; Wang, M.; Li, C.X.; Li, C.J. Ultra-high open-circuit voltage of perovskite solar cells induced by nucleation thermodynamics on rough substrates. Sci. Rep. 2017, 7, 46141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.M.; Zhu, W.D.; Bao, C.X.; Yu, T.; Wang, Y.Q.; Zhou, X.X.; Zou, Z.G. Laser-assisted crystallization of CH3NH3PbI3 films for efficient perovskite solar cells with a high open-circuit voltage. Chem. Commun. 2016, 52, 5394–5397. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Ogomi, Y.; Chang, J.; Tsukamoto, S.; Kukihara, K.; Oshima, T.; Osada, N.; Yoshino, K.; Katayama, K.; Toyoda, T.; et al. Charge transfer and recombination at the metal oxide/CH3NH3PbClI2/spiro-OMeTAD interfaces: Uncovering the detailed mechanism behind high efficiency solar cells. Phys. Chem. Chem. Phys. 2014, 16, 19984–19992. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wang, Y.; Zhu, Q.; Wang, N.; Zhu, D.; Wang, J.; Yang, A.; Yang, R. Efficient planar perovskite solar cells with large fill factor and excellent stability. J. Power Sources 2015, 297, 53–58. [Google Scholar] [CrossRef]
- Ding, B.; Gao, L.; Liang, L.; Chu, Q.; Song, X.; Li, Y.; Yang, G.; Fan, B.; Wang, M.; Li, C. Facile and scalable fabrication of highly efficient lead iodide perovskite thin-film solar cells in air using gas pump method. ACS Appl. Mater. Interfaces 2016, 8, 20067. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.L.; Chen, C.P.; Chang, J.Y.; Yu, Y.Y.; Shen, Y.K. Two-step thermal annealing improves the morphology of spin-coated films for highly efficient perovskite hybrid photovoltaics. Nanoscale 2014, 6, 10281–10288. [Google Scholar] [CrossRef] [PubMed]
- Barrows, A.T.; Pearson, A.J.; Kwak, C.K.; Dunbar, A.D.F.; Buckley, A.R.; Lidzey, D.G. Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition. Energy Environ. Sci. 2014, 7, 2944–2950. [Google Scholar] [CrossRef]
- Heo, J.H.; Song, D.H.; Im, S.H. Planar CH3NH3PbBr3 Hybrid Solar Cells with 10.4% Power Conversion Efficiency, Fabricated by Controlled Crystallization in the Spin-Coating Process. Adv. Mater. 2014, 26, 8179–8183. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.K.; Mathew, C.T.; Koshy, J.; Solomon, S. Influence of La3+ ion in the yttria matrix in improving the microhardness of infrared transparent nano LaxY2-xO3 sintered via hybrid heating. J. Adv. Ceram. 2017, 6, 240–250. [Google Scholar] [CrossRef]
- Clyne, T.W.; Golosnoy, I.O.; Tan, J.C.; Markaki, A.E. Porous materials for thermal management under extreme conditions. Philos. Trans. A Math Phys. Eng. Sci. 2006, 364, 125–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, M.; Fang, Y.; Yuan, J.; Zhu, Y.; Liu, Q.; Song, J. F-doped TiO2 compact film for high-efficient perovskite solar cells. Int. J. Electrochem. Sci. 2017, 12, 1064–1074. [Google Scholar] [CrossRef]
- Chen, J.; Wan, Z.; Liu, J.; Fu, S.-Q.; Zhang, F.; Yang, S.; Tao, S.; Wang, M.; Chen, C. Growth of compact CH3NH3PbI3 thin films governed by the crystallization in PbI2 matrix for efficient planar perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 8649–8658. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, P.; Zhang, Y.; Gu, H.; Cai, Q.; Liu, X.; Wang, J.; Wen, H.; Shao, G. Mild solution-processed metal-doped TiO2 compact layers for hysteresis-less and performance-enhanced perovskite solar cells. J. Power Sources 2017, 372, 235–244. [Google Scholar] [CrossRef]
- Rajmohan, G.D.; Huang, F.Z.; d’Agostino, R.; du Plessis, J.; Dai, X.J. Low temperature reactively sputtered crystalline TiO2 thin film as effective blocking layer for perovskite solar cells. Thin Solid Films 2017, 636, 307–313. [Google Scholar] [CrossRef]
- Ren, Z.; Zhu, M.; Li, X.; Dong, C. An isopropanol-assisted fabrication strategy of pinhole-free perovskite films in air for efficient and stable planar perovskite solar cells. J. Power Sources 2017, 363, 317–326. [Google Scholar] [CrossRef]
- Ye, T.; Xing, J.; Petrovic, M.; Chen, S.; Chellappan, V.; Subramanian, G.S.; Sum, T.C.; Liu, B.; Xiong, Q.; Ramakrishna, S. Temperature effect of the compact TiO2 layer in planar perovskite solar cells: An interfacial electrical, optical and carrier mobility study. Sol. Energy Mater. Sol. Cells 2017, 163, 242–249. [Google Scholar] [CrossRef]
- Zardetto, V.; Di Giacomo, F.; Lifka, H.; Verheijen, M.A.; Weijtens, C.H.L.; Black, L.E.; Veenstra, S.; Kessels, W.M.M.; Andriessen, R.; Creatore, M. Surface fluorination of ALD TiO2 electron transport layer for efficient planar perovskite solar cells. Adv. Mater. Interfaces 2018, 5, 1701456. [Google Scholar] [CrossRef]
- Zardetto, V.; Di Giacomo, F.; Lucarelli, G.; Kessels, W.M.M.; Brown, T.M.; Creatore, M. Plasma-assisted atomic layer deposition of TiO2 compact layers for flexible mesostructured perovskite solar cells. Sol. Energy 2017, 150, 447–453. [Google Scholar] [CrossRef]
- Liu, D.Y.; Yang, J.L.; Kelly, T.L. Compact layer free perovskite solar cells with 13.5% efficiency. J. Am. Chem. Soc. 2014, 136, 17116–17122. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Zheng, E.Q.; Wang, X.F.; Tian, W.J.; Miyasaka, T. Low-temperature-processed ZnO-SnO2 nanocomposite for efficient planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 144, 623–630. [Google Scholar] [CrossRef]
- Adachi, M.; Sakamoto, M.; Jiu, J.T.; Ogata, Y.; Isoda, S. Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy. J. Phys. Chem. B 2006, 110, 13872–13880. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J.; Fabregat-Santiago, F.; Mora-Sero, I.; Garcia-Belmonte, G.; Gimenez, S. Electron lifetime in dye-sensitized solar cells: Theory and interpretation of measurements. J. Phys. Chem. C 2009, 113, 17278–17290. [Google Scholar] [CrossRef]






| Cell type | Jsc/mA·cm−2 | Voc/V | FF/% | η/% | τr/ms |
|---|---|---|---|---|---|
| Monolayer PSCs | 20.78 | 1.09 | 71.74 | 16.25 | 0.45 |
| Bilayer PSCs | 20.10 | 1.09 | 64.99 | 14.24 | 0.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, X.; Chu, Q.; Dong, H.; Yao, J.; Zhou, Y.; Yang, G. Tuning Nucleation Sites to Enable Monolayer Perovskite Films for Highly Efficient Perovskite Solar Cells. Coatings 2018, 8, 408. https://doi.org/10.3390/coatings8110408
Li Y, Li X, Chu Q, Dong H, Yao J, Zhou Y, Yang G. Tuning Nucleation Sites to Enable Monolayer Perovskite Films for Highly Efficient Perovskite Solar Cells. Coatings. 2018; 8(11):408. https://doi.org/10.3390/coatings8110408
Chicago/Turabian StyleLi, Yan, Xiaolei Li, Qianqian Chu, Hui Dong, Jiantao Yao, Yong Zhou, and Guanjun Yang. 2018. "Tuning Nucleation Sites to Enable Monolayer Perovskite Films for Highly Efficient Perovskite Solar Cells" Coatings 8, no. 11: 408. https://doi.org/10.3390/coatings8110408
APA StyleLi, Y., Li, X., Chu, Q., Dong, H., Yao, J., Zhou, Y., & Yang, G. (2018). Tuning Nucleation Sites to Enable Monolayer Perovskite Films for Highly Efficient Perovskite Solar Cells. Coatings, 8(11), 408. https://doi.org/10.3390/coatings8110408