Surface Modification on Cellulose Nanofibers by TiO2 Coating for Achieving High Capture Efficiency of Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of TiO2-Coated Cellulose Nanofibers
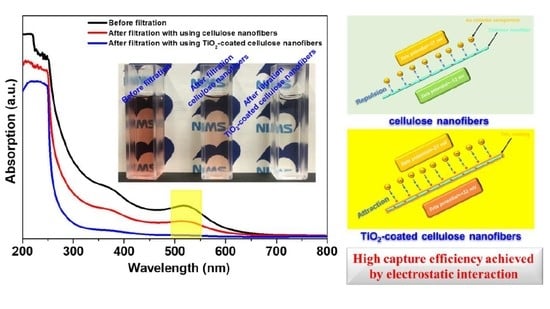
2.2. Evaluation of Capture Efficiency for Au Nanoparticles
2.3. Characterizations
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Osada, Y.; Nakagawa, T. Membrane Science and Technology; Marcel Dekker: New York, NY, USA, 1992. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; Wiley: West Sussex, UK, 2004. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers’ Handbook; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Kontturi, K.S.; Biegaj, K.; Mautner, A.; Woodward, R.T.; Wilson, B.P.; Johansson, L.S.; Lee, K.Y.; Heng, J.Y.Y.; Bismarck, A.; Kontturi, E. Noncovalent surface modification of cellulose nanopapers by adsorption of polymers from aprotic solvents. Langmuir 2017, 33, 5707–5712. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Askeland, P.; Drzal, L.T. Surface modification of microfibrillated cellulose for epoxy composite applications. Polymer 2008, 49, 1285–1296. [Google Scholar] [CrossRef]
- Morandi, G.; Heath, L.; Thielemans, W. Cellulose nanocrystals grafted with polystyrene chains through surface-initiated atom transfer radical polymerization (SI-ATRP). Langmuir 2009, 25, 8280–8286. [Google Scholar] [CrossRef]
- Huang, J.; Kunitake, T. Nano-precision replication of natural cellulosic substances by metal oxides. J. Am. Chem. Soc. 2003, 125, 11834–11835. [Google Scholar] [CrossRef]
- Huang, J.; Matsunaga, N.; Shimanoe, K.; Yamazoe, N.; Kunitake, T. Nanotubular SnO2 templated by cellulose fibers: Synthesis and gas sensing. Chem. Mater. 2005, 17, 3513–3518. [Google Scholar] [CrossRef]
- Huang, J.; Kunitake, T.; Onoue, S.Y. A facile route to a highly stabilized hierarchical hybrid of titania nanotube and gold nanoparticle. Chem. Commun. 2004, 1008–1009. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Kunitake, T. Biomolecular modification of hierarchical cellulose fibers through titania nanocoating. Angew. Chem. 2006, 118, 2949–2952. [Google Scholar] [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.S.W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Figoli, A.; Hoinkis, J.; Altinkaya, S.A.; Bundschuh, J. Application of Nanotechnology in Membranes for Water Treatment; CRC Press: London, UK, 2017. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Handbook of Polymers for Pharmaceutical Technologies, Processing and Applications; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Ichinose, I.; Senzu, H.; Kunitake, T. A surface sol−gel process of TiO2 and other metal oxide films with molecular precision. Chem. Mater. 1997, 9, 1296–1298. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Kunitake, T. Replication of dendrimer monolayer as nanopores in titania ultrathin film. Chem. Commun. 2002, 18, 2070–2071. [Google Scholar] [CrossRef]
- Sobsey, M.D.; Jones, B.L. Concentration of poliovirus from tap water using positively charged microporous filters. Appl. Environ. Microbiol. 1979, 37, 588–595. [Google Scholar]
- Michen, B.; Graule, T. Isoelectric points of viruses. J. Appl. Microbiol. 2010, 109, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Wei, X.; Wang, F.; Han, D.; Wang, Q.; Kong, L. Homogeneous isolation of nanocelluloses by controlling the shearing force and pressure in microenvironment. Carbohydr. Polym. 2014, 113, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Ismail, H.; Rozman, H.D.; Ahmad, M.N. The effect of acetylation on interfacial shear strength between plant fibres and various matrices. Eur. Polym. J. 2001, 37, 1037–1045. [Google Scholar] [CrossRef]
- Wandlowski, T.; Ataka, K.; Pronkin, S.; Diesing, D. Surface enhanced infrared spectroscopy—Au(1 1 1-20 nm)/sulphuric acid—New aspects and challenges. Electrochim. Acta 2004, 49, 1233–1247. [Google Scholar] [CrossRef]
- Åkerholm, M.; Salmén, L. Interactions between wood polymers studied by dynamic FT-IR spectroscopy. Polymer 2001, 42, 963–969. [Google Scholar] [CrossRef]
- Liu, Y. Recent progress in fourier transform infrared (FTIR) spectroscopy study of compositional, structural and physical attributes of developmental cotton fibers. Materials 2013, 6, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chiang, B.S.; Chang, S.; Liu, D.S. Determination of photocatalytic activity in amorphous and crystalline titanium oxide films prepared using plasma-enhanced chemical vapor deposition. Appl. Surf. Sci. 2011, 257, 1893–1897. [Google Scholar] [CrossRef]
- Lee, W.G.; Won, S.I.; Kim, J.C.; Choi, S.H.; Oh, K.H. Preparation and properties of amorphous TiO2 thin films by plasma enhanced chemical vapor deposition. Thin Solid Films 1994, 237, 105–111. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- Butt, H.J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Pujar, N.S.; Zydney, A.L. Electrostatic and electrokinetic interactions during protein transport through narrow pore membranes. Ind. Eng. Chem. Res. 1994, 33, 2473–2482. [Google Scholar] [CrossRef]
- Van Eijndhoven, R.H.; Saksena, S.; Zydney, A.L. Protein fractionation using electrostatic interactions in membranel filtration. Biotechnol. Bioeng. 1995, 48, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Van Reis, R.; Gadam, S.; Frautschy, L.N.; Orlando, S.; Goodrich, E.M.; Saksena, S.; Kuriyel, R.; Simpson, C.M.; Pearl, S.; Zydney, A.L. High performance tangential flow filtration. Biotechnol. Bioeng. 1997, 56, 71–82. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Role of electrostaticinteractionsin the retention of pharmaceuticallyactive contaminantsby a loose nanofiltration membrane. J. Membr. Sci. 2006, 286, 52–59. [Google Scholar] [CrossRef]
- Cho, J.; Amy, G.; Pellegrino, J. Membrane filtration of natural organic matter: factors and mechanisms affecting rejection and flux decline with charged ultrafiltration (UF) membrane. J. Membr. Sci. 2000, 164, 89–110. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. Tailoring membrane surface charges: A novel study on electrostatic interactions during membrane fouling. Polymers 2015, 7, 2017–2030. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Uchikoshi, T.; Ichinose, I.; Liu, L. Surface Modification on Cellulose Nanofibers by TiO2 Coating for Achieving High Capture Efficiency of Nanoparticles. Coatings 2019, 9, 139. https://doi.org/10.3390/coatings9020139
Zhang C, Uchikoshi T, Ichinose I, Liu L. Surface Modification on Cellulose Nanofibers by TiO2 Coating for Achieving High Capture Efficiency of Nanoparticles. Coatings. 2019; 9(2):139. https://doi.org/10.3390/coatings9020139
Chicago/Turabian StyleZhang, Chenning, Tetsuo Uchikoshi, Izumi Ichinose, and Lihong Liu. 2019. "Surface Modification on Cellulose Nanofibers by TiO2 Coating for Achieving High Capture Efficiency of Nanoparticles" Coatings 9, no. 2: 139. https://doi.org/10.3390/coatings9020139
APA StyleZhang, C., Uchikoshi, T., Ichinose, I., & Liu, L. (2019). Surface Modification on Cellulose Nanofibers by TiO2 Coating for Achieving High Capture Efficiency of Nanoparticles. Coatings, 9(2), 139. https://doi.org/10.3390/coatings9020139