Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Changes in Body Weight
2.3. Open Field Test
2.4. Rotarod
2.5. Stride Length
2.6. Sample Collection
2.7. Immunohistochemistry (IHC)
2.8. Histological Analysis
2.8.1. Counting of TH-Positive Cells in the Substantia Nigra
2.8.2. Measurement of TH-Positive Fibers Density in the Striatum
2.9. Statistical Analysis
3. Results
3.1. Changes in Body Weight
3.2. Rotarod
3.3. Stride Length
3.4. Open Field Test
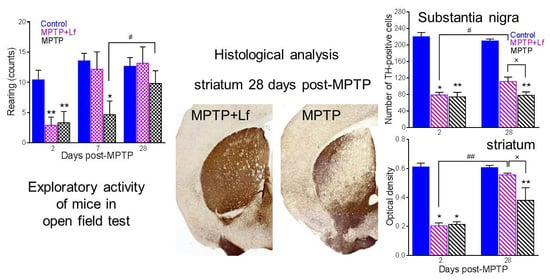
3.5. Histological Analysis
3.6. Correlation between the Number of Rearings in OF Test and Number of TH-Positive Immunoreactive Neurons and Fibers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Simonian, N.A. Oxidative Stress in Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Ramonet, D.; Perier, C. Mitochondrial alterations in Parkinson’s disease: New clues: Mitochondrial alterations in Parkinson’s disease. J. Neurochem. 2008, 107, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.K.; Kim, D.W.; Kim, S.T.; Park, S.Y.; Yeo, E.J.; Choi, Y.J.; Lee, H.-R.; Kim, D.-S.; Cho, S.-W.; Han, K.H.; et al. PEP-1-HO-1 prevents MPTP-induced degeneration of dopaminergic neurons in a Parkinson’s disease mouse model. BMB Rep. 2014, 47, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Sedelis, M.; Schwarting, R.K.W.; Huston, J.P. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav. Brain Res. 2001, 125, 109–125. [Google Scholar] [CrossRef]
- Cao, Q.; Qin, L.; Huang, F.; Wang, X.; Yang, L.; Shi, H.; Wu, H.; Zhang, B.; Chen, Z.; Wu, X. Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson’s disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharmacol. 2017, 319, 80–90. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Gubellini, P.; Kachidian, P. Animal models of Parkinson’s disease: An updated overview. Rev. Neurol. 2015, 171, 750–761. [Google Scholar] [CrossRef]
- Knight, J.A. Reactive oxygen species and the neurodegenerative disorders. Ann. Clin. Lab. Sci. 1997, 27, 11–25. [Google Scholar] [PubMed]
- Aguirre-Vidal, Y.; Monroy-Noyola, A.; Anaya-Ramos, L.; Arteaga-Silva, M.; Mendez-Armenta, M.; Ostoa-Saloma, P.; Díaz-Zaragoza, M.; Morales-Montor, J.; Ríos, C.; Montes, S. β-Estradiol-3-benzoate confers neuroprotection in Parkinson MPP + rat model through inhibition of lipid peroxidation. Steroids 2017, 126, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.R.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991, 114, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Huang, J.; Liang, J.; Ma, M.; Zhao, Q.; Lei, X.; Shi, C.; Luo, L. Evaluation of abnormal iron distribution in specific regions in the brains of patients with Parkinson’s disease using quantitative susceptibility mapping and R2* mapping. Exp. Ther. Med. 2020. [Google Scholar] [CrossRef]
- You, L.-H.; Li, F.; Wang, L.; Zhao, S.-E.; Wang, S.-M.; Zhang, L.-L.; Zhang, L.-H.; Duan, X.-L.; Yu, P.; Chang, Y.-Z. Brain iron accumulation exacerbates the pathogenesis of MPTP-induced Parkinson’s disease. Neuroscience 2015, 284, 234–246. [Google Scholar] [CrossRef]
- Xu, S.-F.; Zhang, Y.-H.; Wang, S.; Pang, Z.-Q.; Fan, Y.-G.; Li, J.-Y.; Wang, Z.-Y.; Guo, C. Lactoferrin ameliorates dopaminergic neurodegeneration and motor deficits in MPTP-treated mice. Redox Biol. 2019, 21, 101090. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.H.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef]
- Acevedo, K.; Masaldan, S.; Opazo, C.M.; Bush, A.I. Redox active metals in neurodegenerative diseases. J. Biol. Inorg. Chem. 2019, 24, 1141–1157. [Google Scholar] [CrossRef]
- Johnson, E.E.; Wessling-Resnick, M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012, 14, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Ward, P.P.; Conneely, O.M. Lactoferrin: Role in iron homeostasis and host defense against microbial infection. Biometals 2004, 17, 203–208. [Google Scholar] [CrossRef]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Orsi, N. The antimicrobial activity of lactoferrin: Current status and perspectives. Biometals 2004, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, J.; Qin, L.; Guo, D.; Ding, H.; Deng, D. Radioprotective effect of lactoferrin in mice exposed to sublethal X-ray irradiation. Exp. Ther. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kopaeva, M.Y.; Alchinova, I.B.; Nesterenko, M.V.; Cherepov, A.B.; Zarayskaya, I.Y.; Karganov, M.Y. Lactoferrin beneficially influences the recovery of physiological and behavioral indexes in mice exposed to acute gamma-irradiation. Patog. Pathog. 2020, 18, 29–33. [Google Scholar] [CrossRef]
- Leveugle, B.; Faucheux, B.A.; Bouras, C.; Nillesse, N.; Spik, G.; Hirsch, E.C.; Agid, Y.; Hof, P.R. Cellular distribution of the iron-binding protein lactotransferrin in the mesencephalon of Parkinson’s disease cases. Acta Neuropathol. 1996, 91, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, B.A.; Nillesse, N.; Damier, P.; Spik, G.; Mouatt-Prigent, A.; Pierce, A.; Leveugle, B.; Kubis, N.; Hauw, J.J.; Agid, Y. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1995, 92, 9603–9607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A.; Wang, B. Lactoferrin Promotes Early Neurodevelopment and Cognition in Postnatal Piglets by Upregulating the BDNF Signaling Pathway and Polysialylation. Mol. Neurobiol. 2015, 52, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Wang, B. Molecular Determinants of Milk Lactoferrin as a Bioactive Compound in Early Neurodevelopment and Cognition. J. Pediatr. 2016, 173, S29–S36. [Google Scholar] [CrossRef] [Green Version]
- Fillebeen, C.; Descamps, L.; Dehouck, M.-P.; Fenart, L.; Benaїssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [Green Version]
- Kamemori, N.; Takeuchi, T.; Sugiyama, A.; Miyabayashi, M.; Kitagawa, H.; Shimizu, H.; Ando, K.; Harada, E. Trans-Endothelial and Trans-Epithelial Transfer of Lactoferrin into the Brain through BBB and BCSFB in Adult Rats. J. Vet. Med. Sci. 2008, 70, 313–315. [Google Scholar] [CrossRef] [Green Version]
- Kopaeva, Y.; Cherepov, A.B.; Zarayskaya, I.Y.; Nesterenko, M.V. Transport of Human Lactoferrin into Mouse Brain: Administration Routes and Distribution. Bull. Exp. Biol. Med. 2019, 167, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Ferger, B. The Protective Effects of PBN Against MPTP Toxicity Are Independent of Hydroxyl Radical Trapping. Pharmacol. Biochem. Behav. 2000, 65, 425–431. [Google Scholar] [CrossRef]
- Faraji, N.; Zhang, Y.; Ray, A.K. Determination of adsorption isotherm parameters for minor whey proteins by gradient elution preparative liquid chromatography. J. Chromatogr. A 2015, 1412, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Hassan, M.I.; Kashav, T.; Singh, T.P.; Yadav, S. Heparin-binding proteins of human seminal plasma: Purification and characterization. Mol. Reprod. Dev. 2008, 75, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lou, F.; Wu, W.; Dong, X.; Ren, J.; Shen, Q. Determination of Bovine Lactoferrin in Food by HPLC with a Heparin Affinity Column for Sample Preparation. J. AOAC Int. 2017, 100, 133–138. [Google Scholar] [CrossRef] [PubMed]
- On Protection of Animals Used for Scientific Purposes: Education and Training; Framework National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes // Brussels. 19–20 February 2014. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/guidance/education_training/en.pdf (accessed on 14 December 2020).
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice; Gould, T.D., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. ISBN 978-1-60761-302-2. [Google Scholar]
- Brooks, S.P.; Dunnett, S.B. Tests to assess motor phenotype in mice: A user’s guide. Nat. Rev. Neurosci. 2009, 10, 519–529. [Google Scholar] [CrossRef]
- Fernagut, P.O.; Diguet, E.; Labattu, B.; Tison, F. A simple method to measure stride length as an index of nigrostriatal dysfunction in mice. J. Neurosci. Methods 2002, 113, 123–130. [Google Scholar] [CrossRef]
- Fleming, S.M. Early and Progressive Sensorimotor Anomalies in Mice Overexpressing Wild-Type Human -Synuclein. J. Neurosci. 2004, 24, 9434–9440. [Google Scholar] [CrossRef] [Green Version]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 2008. [Google Scholar]
- Jackson-Lewis, V.; Jakowec, M.; Burke, R.E.; Przedborski, S. Time Course and Morphology of Dopaminergic Neuronal Death Caused by the Neurotoxin 1-methyl-4-phenyl-l,2,3,6-tetrahydropyridine. Neurodegeneration 1995, 4, 257–269. [Google Scholar] [CrossRef]
- Mengual, E.; Pickel, V.M. Regional and subcellular compartmentation of the dopamine transporter and tyrosine hydroxylase in the rat ventral pallidum. J. Comp. Neurol. 2004, 468, 395–409. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Sarre, S.; Ebinger, G.; Michotte, Y. Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J. Neurosci. Methods 2005, 144, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.I.; Duarte-Silva, S.; Silva-Fernandes, A.; Nunes, M.J.; Carvalho, A.N.; Rodrigues, E.; Gama, M.J.; Rodrigues, C.M.P.; Maciel, P.; Castro-Caldas, M. Tauroursodeoxycholic Acid Improves Motor Symptoms in a Mouse Model of Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 9139–9155. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, A.; Plaznik, A.; Sundström, E.; Jonsson, G.; Archer, T. MPTP-Induced Hypoactivity in Mice: Reversal by L-Dopa. Pharmacol. Toxicol. 1990, 67, 295–301. [Google Scholar] [CrossRef]
- Fredriksson, A.; Archer, T. MPTP-induced behavioural and biochemical deficits: A parametric analysis. J. Neural Transm. Gen. Sect. 1994, 7, 123–132. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Sedelis, M.; Hofele, K.; Auburger, G.W.; Huston, J.P. Strain-dependent recovery of open-field behavior and striatal dopamine deficiency in the mouse MPTP model of Parkinson’s disease. Neurotox. Res. 1999, 1, 41–56. [Google Scholar] [CrossRef]
- Sundström, E.; Fredriksson, A.; Archer, T. Chronic neurochemical and behavioral changes in MPTP-lesioned C57BL/6 mice: A model for Parkinson’s disease. Brain Res. 1990, 528, 181–188. [Google Scholar] [CrossRef]
- Salamone, J.D.; Pardo, M.; Yohn, S.E.; López-Cruz, L.; SanMiguel, N.; Correa, M. Mesolimbic Dopamine and the Regulation of Motivated Behavior. In Behavioral Neuroscience of Motivation; Simpson, E.H., Balsam, P.D., Eds.; Current Topics in Behavioral Neurosciences; Springer International Publishing: Cham, Switzerland, 2015; Volume 27, pp. 231–257. ISBN 978-3-319-26933-7. [Google Scholar]
- Liu, H.; Wu, H.; Zhu, N.; Xu, Z.; Wang, Y.; Qu, Y.; Wang, J. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J. Neurochem. 2020, 152, 397–415. [Google Scholar] [CrossRef]
- Saporito, M.S.; Brown, E.M.; Miller, M.S.; Carswell, S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J. Pharmacol. Exp. Ther. 1999, 288, 421. [Google Scholar]
- Jakowec, M.W.; Nixon, K.; Hogg, E.; McNeill, T.; Petzinger, G.M. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of the mouse nigrostriatal pathway. J. Neurosci. Res. 2004, 76, 539–550. [Google Scholar] [CrossRef]
- Depboylu, C.; Schorlemmer, K.; Klietz, M.; Oertel, W.H.; Weihe, E.; Höglinger, G.U.; Schäfer, M.K.-H. Upregulation of microglial C1q expression has no effects on nigrostriatal dopaminergic injury in the MPTP mouse model of Parkinson disease. J. Neuroimmunol. 2011, 236, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cawthon, D.; McCastlain, K.A.; Slikker, W.; Ali, S.F. Selective alterations of gene expression in mice induced by MPTP. Synapse 2005, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, P.; Mena, N.P.; Carrasco, C.M.; Muñoz, Y.; Pérez-Henríquez, P.; Morales, R.A.; Cassels, B.K.; Méndez-Gálvez, C.; García-Beltrán, O.; González-Billault, C.; et al. Iron chelators and antioxidants regenerate neuritic tree and nigrostriatal fibers of MPP+/MPTP-lesioned dopaminergic neurons. PLoS ONE 2015, 10, e0144848. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Dovero, S.; Imbert, C.; Boraud, T.; Gross, C.E. Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. Synapse 2000, 38, 363–368. [Google Scholar] [CrossRef]
- Kolacheva, A.A.; Ugrumov, M.V. Dopamine synthesis as a mechanism of brain plasticity in nigrostriatal system pathology. Dokl. Biochem. Biophys. 2018, 479, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Depboylu, C.; Klietz, M.; Maurer, L.; Oertel, W.H.; Kobayashi, K.; Weihe, E.; Höglinger, G.U.; Schäfer, M.K.-H. Transcriptional and structural plasticity of tyrosine hydroxylase expressing neurons in both striatum and nucleus accumbens following dopaminergic denervation. J. Chem. Neuroanat. 2014, 61–62, 169–175. [Google Scholar] [CrossRef]
- Ziere, G.J.; van Dijk, M.C.; Bijsterbosch, M.K.; van Berkel, T.J. Lactoferrin uptake by the rat liver. Characterization of the recognition site and effect of selective modification of arginine residues. J. Biol. Chem. 1992, 267, 11229–11235. [Google Scholar]
- Rousseau, E.; Michel, P.P.; Hirsch, E.C. The Iron-Binding Protein Lactoferrin Protects Vulnerable Dopamine Neurons from Degeneration by Preserving Mitochondrial Calcium Homeostasis. Mol. Pharmacol. 2013, 84, 888–898. [Google Scholar] [CrossRef] [Green Version]
- Rozas, G.; López-Martín, E.; Guerra, M.J.; Labandeira-García, J.L. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods 1998, 83, 165–175. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopaeva, M.Y.; Cherepov, A.B.; Nesterenko, M.V.; Zarayskaya, I.Y. Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology 2021, 10, 24. https://doi.org/10.3390/biology10010024
Kopaeva MY, Cherepov AB, Nesterenko MV, Zarayskaya IY. Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology. 2021; 10(1):24. https://doi.org/10.3390/biology10010024
Chicago/Turabian StyleKopaeva, Marina Yu., Anton B. Cherepov, Mikhail V. Nesterenko, and Irina Yu. Zarayskaya. 2021. "Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure" Biology 10, no. 1: 24. https://doi.org/10.3390/biology10010024
APA StyleKopaeva, M. Y., Cherepov, A. B., Nesterenko, M. V., & Zarayskaya, I. Y. (2021). Pretreatment with Human Lactoferrin Had a Positive Effect on the Dynamics of Mouse Nigrostriatal System Recovery after Acute MPTP Exposure. Biology, 10(1), 24. https://doi.org/10.3390/biology10010024