Do Females in a Unisexual-Bisexual Species Complex Differ in Their Behavioral Syndromes and Cortisol Production?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Maintenance
2.2. Identification of Behavioral Types
2.3. Hormone Sampling
2.4. Statistical Analysis
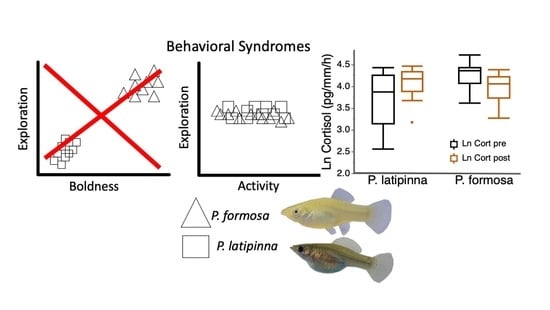
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sih, A.; Bell, A.M.; Johnson, J.C.; Ziemba, R.E. Behavioral syndromes: An integrative overview. Q. Rev. Biol. 2004, 79, 241–277. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Conrad, J.L.; Weinersmith, K.L.; Brodin, T.; Saltz, J.B.; Sih, A. Behavioural syndromes in fishes: A review with implications for ecology and fisheries management. J. Fish Biol. 2011, 78, 395–435. [Google Scholar] [CrossRef]
- Réale, D.; Dingemanse, N.J.; Kazem, A.J.N.; Wright, J. Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3937–3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef] [Green Version]
- Roche, D.G.; Careau, V.; Binning, S.A. Demystifying animal ‘personality’ (or not): Why individual variation matters to experimental biologists. J. Exp. Biol. 2016, 219, 3832–3843. [Google Scholar] [CrossRef] [Green Version]
- Koolhaas, J.; De Boer, S.; Coppens, C.; Buwalda, B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front. Neuroendocrinol. 2010, 31, 307–321. [Google Scholar] [CrossRef]
- Santicchia, F.; Wauters, L.A.; Dantzer, B.; Westrick, S.E.; Ferrari, N.; Romeo, C.; Palme, R.; Preatoni, D.G.; Martinoli, A. Relationships between personality traits and the physiological stress response in a wild mammal. Curr. Zool. 2019, 66, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gosling, S.D. From mice to men: What can we learn about personality from animal research? Psychol. Bull. 2001, 127, 45–86. [Google Scholar] [CrossRef] [Green Version]
- Dingemanse, N.J.; Réale, D. Natural selection and animal personality. Behaviour 2005, 142, 1159–1184. [Google Scholar] [CrossRef]
- Bell, A.M.; Hankison, S.J.; Laskowski, K.L. The repeatability of behaviour: A meta-analysis. Anim. Behav. 2009, 77, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Lande, R.; Arnold, S.J. The measurement of selection on correlated characters. Evolution 1983, 37, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Ketterson, E.D.; Nolan, V., Jr. Adaptation, exaptation, and constraint: A hormonal perspective. Am. Nat. 1999, 154, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.M. Future directions in behavioural syndromes research. Proc. Biol. Sci. 2007, 274, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.M. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). J. Evol. Biol. 2005, 18, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Dochtermann, N.A.; Dingemanse, N.J. Behavioral syndromes as evolutionary constraints. Behav. Ecol. 2013, 24, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Cheverud, J.M. Developmental Integration and the Evolution of Pleiotropy. Am. Zool. 1996, 36, 44–50. [Google Scholar] [CrossRef]
- Wilson, D.S. Adaptive individual differences within single populations. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.; Jones, F.; Braithwaite, V. In situ examination of boldness–shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim. Behav. 2005, 70, 1003–1009. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.; Jones, F.; Braithwaite, V.A. Correlation between boldness and body mass in natural populations of the poeciliid Brachyrhaphis episcopi. J. Fish Biol. 2007, 71, 1590–1601. [Google Scholar] [CrossRef] [Green Version]
- Dingemanse, N.J.; Wright, J.; Kazem, A.J.; Thomas, D.K.; Hickling, R.; Dawnay, N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J. Anim. Ecol. 2007, 76, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Royauté, R.; Hedrick, A.; Dochtermann, N.A. Behavioural syndromes shape evolutionary trajectories via conserved genetic architecture. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200183. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.M.; Sih, A. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 2007, 10, 828–834. [Google Scholar] [CrossRef]
- Michelangeli, M.; Chapple, D.G.; Goulet, C.T.; Bertram, M.G.; Wong, B.B.M. Behavioral syndromes vary among geographically distinct populations in a reptile. Behav. Ecol. 2018, 30, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Pruitt, J.N.; Riechert, S.E.; Iturralde, G.; Vega, M.; Fitzpatrick, B.M.; Avilés, L. Population differences in behaviour are explained by shared within-population trait correlations. J. Evol. Biol. 2010, 23, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, R.A.; Sockman, K.W. Proximate mechanisms of behavioural inflexibility: Implications for the evolution of personality traits. Funct. Ecol. 2012, 26, 559–566. [Google Scholar] [CrossRef]
- Garamszegi, L.Z.; Markó, G.; Herczeg, G. A meta-analysis of correlated behaviours with implications for behavioural syndromes: Mean effect size, publication bias, phylogenetic effects and the role of mediator variables. Evol. Ecol. 2012, 26, 1213–1235. [Google Scholar] [CrossRef] [Green Version]
- Carere, C.; Caramaschi, D.; Fawcett, T.W. Covariation between personalities and individual differences in coping with stress: Converging evidence and hypotheses. Curr. Zool. 2010, 56, 728–740. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Milla, S.; Wang, N.; Mandiki, S.N.; Kestemont, P. Corticosteroids: Friends or foes of teleost fish reproduction? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Thomson, J.S.; Watts, P.C.; Pottinger, T.G.; Sneddon, L.U. Physiological and genetic correlates of boldness: Characterising the mechanisms of behavioural variation in rainbow trout, Oncorhynchus Mykiss. Horm. Behav. 2011, 59, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archard, G.A.; Earley, R.L.; Hanninen, A.F.; Braithwaite, V.A. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol. 2012, 26, 637–645. [Google Scholar] [CrossRef] [Green Version]
- Muraco, J.J.; Aspbury, A.S.; Gabor, C.R. Does male behavioral type correlate with species recognition and stress? Behav. Ecol. 2014, 25, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Hubbs, C.; Hubbs, L.C. Apparent parthenogenesis in nature in a form of fish of hybrid origin. Science 1932, 76, 628–630. [Google Scholar] [CrossRef]
- Alberici da Barbiano, L.; Gompert, Z.; Aspbury, A.S.; Gabor, C.R.; Nice, C.C. Population genomics reveals a possible history of backcrossing and recombination in the gynogenetic fish Poecilia formosa. Proc. Natl. Acad. Sci. USA 2013, 110, 13797–13802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foran, C.M.; Ryan, M.J. Female-female competition in a unisexual/bisexual complex of mollies. Copeia 1994, 1994, 504–508. [Google Scholar] [CrossRef]
- Schlupp, I.; Parzefall, J.; Schartl, M. Male mate choice in mixed bisexual/unisexual breeding complexes of Poecilia (Teleostei: Poeciliidae). Ethology 1991, 88, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Scharnweber, K.; Plath, M.; Tobler, M. Examination of boldness traits in sexual and asexual mollies (Poecilia latipinna, P. formosa). Acta Ethol. 2011, 14, 77. [Google Scholar] [CrossRef]
- Seda, J.B.; Childress, M.J.; Ptacek, M.B. Individual variation in male size and behavioral repertoire in the sailfin molly Poecilia latipinna. Ethology 2012, 118, 411–421. [Google Scholar] [CrossRef]
- Brown, W.H. Introduced fish species of the Guadalupe River Basin. Texas J. Sci. 1953, 5, 245–251. [Google Scholar]
- Lorenz, C.; Opitz, R.; Lutz, I.; Kloas, W. Corticosteroids disrupt amphibian metamorphosis by complex modes of action including increased prolactin expression. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2009, 150, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gabor, C.R.; Grober, M.S. A potential role of male and female androgen in species recognition in a unisexual-bisexual mating complex. Horm. Behav. 2010, 57, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Gabor, C.R.; Contreras, A. Measuring water-borne cortisol in Poecilia latipinna: Is the process stressful, can stress be minimized and is cortisol correlated with sex steroid release rates? J. Fish Biol. 2012, 81, 1327–1339. [Google Scholar] [CrossRef]
- Hadfield, J. MCMC Methods for Multi-Response Generalized Linear Mixed Models: The MCMCglmm R Package. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2015. [Google Scholar]
- Warren, W.C.; García-Pérez, R.; Xu, S.; Lampert, K.P.; Chalopin, D.; Stöck, M.; Loewe, L.; Lu, Y.; Kuderna, L.; Minx, P.; et al. Clonal polymorphism and high heterozygosity in the celibate genome of the Amazon molly. Nat. Ecol. Evol. 2018, 2, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Alberici da Barbiano, L.; Aspbury, A.S.; Nice, C.C.; Gabor, C.R. The impact of social context on male mate preference in a unisexual--bisexual mating complex. J. Fish Biol. 2011, 79, 194–204. [Google Scholar] [CrossRef]
- Travis, J.; Woodward, B.D. Social context and courtship flexibility in male sailfin mollies, Poecilia latipinna (Pisces: Poeciliidae). Anim. Behav. 1989, 38, 1001–1011. [Google Scholar] [CrossRef]
- Travis, J.; Trexler, J.C.; Mulvey, M. Multiple paternity and its correlates in female Poecilia latipinna (Poeciliidae). Copeia 1990, 1990, 722–729. [Google Scholar] [CrossRef]
- Schlupp, I.; Marler, C.; Ryan, M.J. Benefit to male sailfin mollies of mating with heterospecific females. Science 1994, 263, 373–374. [Google Scholar] [CrossRef]
- Gumm, J.M.; Gabor, C.R. Asexuals looking for sex: Conflict between species and mate-quality recognition in sailfin mollies (Poecilia latipinna). Behav. Ecol. Sociobiol. 2005, 58, 558–565. [Google Scholar] [CrossRef]
- Pruitt, J.N.; Riechert, S.E.; Jones, T.C. Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim. Behav. 2008, 76, 871–879. [Google Scholar] [CrossRef]
- Castanheira, M.F.; Herrera, M.; Costas, B.; Conceição, L.E.C.; Martins, C.I.M. Can We Predict Personality in Fish? Searching for Consistency over Time and across Contexts. PLoS ONE 2013, 8, e62037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubin-Horth, N.; Deschenes, M.; Cloutier, S. Natural variation in the molecular stress network correlates with a behavioural syndrome. Horm. Behav. 2012, 61, 140–146. [Google Scholar] [CrossRef]

| ICC (r) | 95% CI | Estimate | 95% CI | |
|---|---|---|---|---|
| Activity-among species | 0.12 | 0–0.25 | ||
| P. latipinna | 0.01 | 0–0.22 | 218.84 | 201.46–237.20 |
| P. formosa | 0.27 | 0–0.39 | −13.05 | −36.94–11.23 |
| Exploration-among species | 0.00 | 0–0.18 | ||
| P. latipinna | 0 | 0–0.20 | 25.44 | 21.00–29.58 |
| P. formosa | 0 | 0–0.22 | 0.99 | −1.32–3.71 |
| Boldness-among species | 0.28 | 0.06–0.36 | ||
| P. latipinna | 0.36 | 0–0.35 | 466.92 | 369.33–565.58 |
| P. formosa | 0.34 | <0.001–0.41 | 15.93 | −40.89–73.82 |
| Correlation Estimate | 95% Credible Intervals | |
|---|---|---|
| a. Boldness ~ Activity | ||
| Among species | 0.21 | −0.66–0.96 |
| P. latipinna | 0.28 | −0.60–0.99 |
| P. formosa | 0.05 | −0.80–0.90 |
| b. Boldness ~ Exploration | ||
| P. latipinna | 0.20 | −0.6–10.96 |
| P. formosa | −0.01 | −0.83–0.90 |
| c. Activity ~ Exploration | ||
| P. latipinna | 0.67 | 0.26–1.00 |
| P. formosa | 0.61 | 0.15–0.97 |
| d. Activity ~ Change in cortisol | ||
| P. latipinna | −0.44 | −1.00–1.00 |
| P. formosa | 0.74 | −1.00–1.00 |
| e. Boldness ~ Change in cortisol | ||
| P. latipinna | −0.20 | −1.00–1.00 |
| P. formosa | −0.65 | −1.00–1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraco, J.J., Jr.; Monroe, D.J.; Aspbury, A.S.; Gabor, C.R. Do Females in a Unisexual-Bisexual Species Complex Differ in Their Behavioral Syndromes and Cortisol Production? Biology 2021, 10, 186. https://doi.org/10.3390/biology10030186
Muraco JJ Jr., Monroe DJ, Aspbury AS, Gabor CR. Do Females in a Unisexual-Bisexual Species Complex Differ in Their Behavioral Syndromes and Cortisol Production? Biology. 2021; 10(3):186. https://doi.org/10.3390/biology10030186
Chicago/Turabian StyleMuraco, James J., Jr., Dillon J. Monroe, Andrea S. Aspbury, and Caitlin R. Gabor. 2021. "Do Females in a Unisexual-Bisexual Species Complex Differ in Their Behavioral Syndromes and Cortisol Production?" Biology 10, no. 3: 186. https://doi.org/10.3390/biology10030186
APA StyleMuraco, J. J., Jr., Monroe, D. J., Aspbury, A. S., & Gabor, C. R. (2021). Do Females in a Unisexual-Bisexual Species Complex Differ in Their Behavioral Syndromes and Cortisol Production? Biology, 10(3), 186. https://doi.org/10.3390/biology10030186