Benthic Biodiversity, Carbon Storage and the Potential for Increasing Negative Feedbacks on Climate Change in Shallow Waters of the Antarctic Peninsula
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
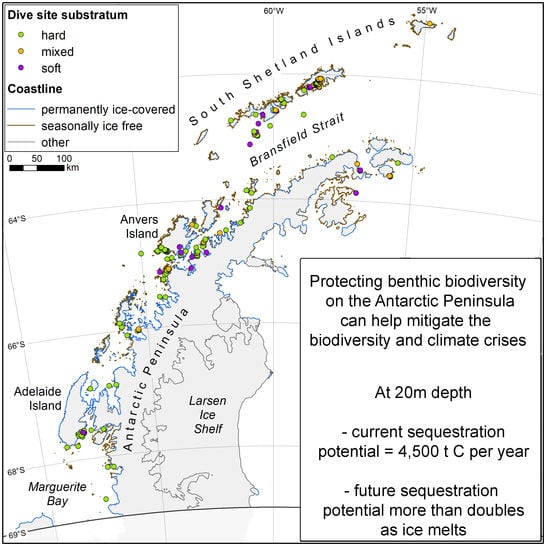
2.1. Study Area
2.2. Study Design
2.3. Modelling Shallow Water Substratum Types
2.4. Estimating Assemblage Carbon Standing Stock and Sequestration
2.5. Statistical Analysis
3. Results
3.1. Hard Substrata Assemblage Structure
3.2. Comparisons of Biomass on Hard and Soft Substrata
3.3. Faunal Assemblage Comparisons
3.4. Functional Groups and Carbon Standing Stock
3.5. Estimating Shallow Benthic Carbon Stores
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Quere, C.; Andrew, R.M.; Friedlingstein, P.; Stitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef] [Green Version]
- Morley, S.A.; Abele, D.; Barnes, D.K.A.; Cárdenas, C.A.; Cotté, C.; Gutt, J.; Henley, S.F.; Höfer, J.; Hughes, K.A.; Martin, S.M.; et al. Global Drivers on Southern Ocean Ecosystems: Changing Physical Environments and Anthropogenic Pressures in an Earth System. Front. Mar. Sci. 2020, 7, 547188. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [CrossRef]
- Erwin, K.L. Wetlands and global climate change: The role of wetland restoration in a changing world. Wetl. Ecol. Manag. 2009, 17, 71–84. [Google Scholar] [CrossRef]
- Krumhansl, K.A.; Okamoto, D.K.; Rassweiler, A.; Novak, M.; Bolton, J.J.; Cavanaugh, K.C.; Connell, S.D.; Johnson, C.R.; Konar, B.; Ling, S.D.; et al. Global patterns of kelp forest change. Proc. Natl. Acad. Sci. USA 2016, 113, 13785–13790. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Jiang, J.; Xue, L.; Craft, C.B. Distribution of organic carbon storage in different salt-marsh plant communities: A case study at the Yangtze Estuary. Estuar. Coast. Shelf Sci. 2020, 243, 106900. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.; Warren, M.W.; Sasmito, S.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The potential of Indonesian mangrove forests for global climate change mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyamik, S.; Calladine, A.; Fourgurean, J.W.; Heck Jnr, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Barnes, D.K.A.; Fleming, A.; Sands, C.J.; Quartino, M.L.; Deregibus, D. Icebergs, sea ice, blue carbon and Antarctic climate feedbacks. Phil. Trans. R. Soc. A 2018, 376, 2122. [Google Scholar] [CrossRef] [Green Version]
- Sala, E.; Mayorga, J.; Bradley, D.; Cabral, R.B.; Atwood, T.B.; Auber, A.; Cheung, W.; Costello, C.; Ferretti, F.; Friedlander, A.M. Protecting the global ocean for biodiversity, food and climate. Nature 2021, 592, 397–402. [Google Scholar] [CrossRef]
- Hilmi, N.; Chami, R.; Sutherland, M.D.; Hall-Spencer, J.M.; Lebleu, L.; Benitez, M.B.; Levin, L.A. The Role of Blue Carbon in Climate Change Mitigation and Carbon Stock Conservation. Front. Clim. 2021, 3. [Google Scholar] [CrossRef]
- Pineda-Metz, S.E.A.; Gerdes, D.; Richter, C. Benthic fauna declined on a whitening continental shelf. Nat. Comms. 2020, 11, 226. [Google Scholar] [CrossRef]
- Souster, T.A. Marine Biodiversity of Antarctic Hard Rock Communities: Species Biomass and Energy Use. Ph.D. Thesis, The Open University, Milton Keynes, UK, 2017. [Google Scholar]
- Zwerschke, N.; Sands, C.J.; Roman-Gonzalez, A.; Barnes, D.K.A.; Guzzi, A.; Jenkins, S.; Muňoz-Ramírez, C. Quantification of blue carbon pathways contributing to negative feedback on climate change following glacier retreat in West Antarctic fjords. Glob. Chang. Biol. 2021, in press. [Google Scholar] [CrossRef]
- Saban, J.M.; Chapman, M.A.; Taylor, G. FACE facts hold for multiple generations; evidence from natural CO2 springs. Glob. Chang. Biol. 2018, 25, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.R.; Twardowski, M.S. “Breaking” news for the ocean’s carbon budget. Science 2020, 367, 6479. [Google Scholar] [CrossRef]
- Takahashi, T.; Sweeney, C.; Hales, B.; Chipman, D.W.; Newberger, T.; Goddard, J.G.; Iannuzzi, R.A.; Sutherland, S.C. The changing carbon cycle in the southern ocean. Oceanography 2012, 25, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Cook, A.J.; Holland, P.R.; Meredith, M.P.; Murray, T.; Luckman, A.; Vaughan, D.G. Ocean forcing of glacier retreat in the western Antarctic Peninsula. Science 2016, 353, 283–286. [Google Scholar] [CrossRef] [Green Version]
- Arntz, W.E.; Brey, T.; Gallardo, V.A. Antarctic zoobenthos. Oceanogr. Mar. Biol. 1994, 32, 241–304. [Google Scholar]
- Barnes, D.K.A.; Brockington, S. Benthic biodiversity, biomass and abundance at Adelaide Island, Antarctica. Mar. Ecol. Prog. Ser. 2003, 249, 145–155. [Google Scholar] [CrossRef]
- Rovelli, L.; Attard, K.M.; Cárdenas, C.A.; Glud, R.N. Benthic primary production and respiration of shallow rocky habitats: A case study from South Bay (Doumer Island, Western Antarctic Peninsula). Pol. Biol. 2019, 42, 1459–1474. [Google Scholar] [CrossRef] [Green Version]
- Pasotti, F.; Saravia, L.A.; De Troch, M.; Tarantelli, M.S.; Sahade, R.; Vanreusel, A. Benthic Trophic Interactions in an Antarctic Shallow Water Ecosystem Affected by Recent Glacier Retreat. PLoS ONE 2015, 10, e0141742. [Google Scholar] [CrossRef] [Green Version]
- Vause, B.J.; Morley, S.A.; Fonseca, V.G.; Jaźdźewska, A.; Ashton, G.V.; Barnes, D.K.A.; Giebner, H.; Clark, M.S.; Peck, L.S. Spatial and temporal dynamics of Antarctic shallow soft-bottom benthic communities: Ecological drivers under climate change. BMC Ecol. 2019, 19, 27. [Google Scholar] [CrossRef] [Green Version]
- Mystikou, A.; Peters, A.; Asensi, A.O.; Fletcher, K.I.; Brickle, P.; van West, P.; Convey, P.; Küpper, F.C. Seaweed biodiversity in the south-western Antarctic Peninsula macroalgal community composition in the Adelaide Island/Marguerite Bay region over a 35-year time span. Polar Biol. 2014, 37, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Amsler, C.D.; Rowley, R.; Laur, D.R.; Quetin, L.B.; Ross, R.M. Vertical distribution of Antarctic peninsular macroalgae: Cover, biomass and species composition. Phycologia 1995, 34, 424–430. [Google Scholar] [CrossRef]
- Klöser, H.; Quartino, M.L.; Wiencke, C. Distribution of macroalgae and macroalgal communities in gradients of physical conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 1996, 333, 1–17. [Google Scholar] [CrossRef]
- Lee, J.R.; Raymond, B.; Bracegirdle, T.J.; Chadès, I.; Fuller, R.A.; Shaw, J.D.; Terauds, A. Climate change drives expansion of Antarctic ice-free habitats. Nature 2017, 547, 49–57. [Google Scholar] [CrossRef]
- Fauchald, K. The polychaete worms: Definitions and keys to the orders, families and genera. Natural history museum of Los Angeles county. Sci. Ser. 1977, 28, 1–188. [Google Scholar]
- Dell, R.K. Antarctic Mollusca: With Special Reference to the Fauna of the Ross Sea; The Royal Society of New Zealand: Wellington, New Zealand, 1990; 311p. [Google Scholar]
- Linse, K. The Shelled Magellanic Mollusca: With Special Reference to Biogeographic Relations in the Southern Ocean; Fricke, R., Ed.; A.R.G. Gantner Verlag KG: Ruggell, Lichenstein, 2002; 252p. [Google Scholar]
- Brueggeman, P. Echonodermata- Asteroidea: Seastars, Underwater field guide to Ross Island & Mcmurdo Sound, Antarctica. Field Guide, 1998. Available online: http://peterbrueggeman.com/nsf/fguide/echinodermata.pdf (accessed on 20 May 2018).
- Hayward, P.J. Antarctic Cheilostomatous Bryozoa; Oxford University Press: Oxford, UK, 1995; 368p. [Google Scholar]
- Barnes, D.K.A.; Sands, C.J.; Richardson, A.; Smith, N. Extremes in Benthic Ecosystem Services; Blue Carbon Natural Capital Shallower than 1000 m in isolated, small and young Ascension Island’s EEZ. Front. Mar. Sci. 2019, 6, 663. [Google Scholar] [CrossRef] [Green Version]
- Souster, T.A.; Barnes, D.K.A.; Hopkins, J. Variation in zoobenthic blue carbon in the Arctic’s Barents Sea shelf sediments. Phil. Trans. Soc. A 2020, 378, 20190362. [Google Scholar] [CrossRef]
- Morley, S.A.; (British Antarctic Survey, Cambridge, Cambridgeshire, UK); Barnes, D.K.A.; (British Antarctic Survey, Cambridge, Cambridgeshire, UK). Personal communication, 2020.
- Weykham, G.; Gómez, I.; Wiencke, C.; Iken, K.; Klöser, H. Photosynthetic characteristics and C:N ratios of macroalgae from King George Island (Antarctica). J. Exp. Mar. Biol. Ecol. 1996, 204, 1–22. [Google Scholar] [CrossRef]
- Krumhansl, K.; Scheibling, R. Production and fate of kelp detritus. Mar. Ecol. Prog. Ser. 2012, 467, 281–302. [Google Scholar] [CrossRef] [Green Version]
- Runcie, J.W.; Riddle, M.J. Estimating primary productivity of marine macroalgae in east Antarctica using in situ fluorometry. Eur. J. Phycol. 2012, 47, 449–460. [Google Scholar] [CrossRef]
- Hardison, A.K.; Canuel, E.A.; Anderson, I.C.; Veuger, B. Fate of macroalgae in benthic systems: Carbon and nitrogen cycling within the microbial community. Mar. Ecol. Prog. Ser. 2010, 414, 41–55. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–743. [Google Scholar] [CrossRef]
- Barnes, D.K.A. Polar zoobenthos blue carbon storage increases with sea ice losses, because across–shelf growth gains from longer algal blooms outweigh ice scour mortality in the shallows. Glob. Chang. Biol. 2017, 23, 5083–5091. [Google Scholar] [CrossRef]
- Schoening, T.; Bergmann, M.; Ontrup, J.; Taylor, J.; Dannheim, J.; Gutt, J.; Purser, A.; Nattkemper, T. Semi-Automated Image Analysis for the Assessment of Megafaunal Densities at the Arctic Deep-Sea Observatory HAUSGARTEN. PLoS ONE 2012, 7, e38179. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Berg, S.; Jivcov, S.; Kusch, S.; Kuhn, G.; White, D.; Bohrmann, G.; Melles, M.; Rethemeyer, R. Increased petrogenic and biospheric organic carbon burial in sub-Antarctic fjord sediments in response to recent glacier retreat. Limnol. Oceanogr. 2021, 66, 4347–4362. [Google Scholar] [CrossRef]
- Peck, L.S. Antarctic marine biodiversity: Adaptations, environments and responses to change. Oceanogr. Mar. Biol. 2018, 56, 105–236. [Google Scholar]
- Valdivia, N.; Garrido, I.; Bruning, P.; Piňones, A.; Pardo, L.M. Biodiversity of an Antarctic subtidal community and its relationship with glacier meltdown processes. Mar. Environ. Res. 2020, 159, 104991. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.J.O.; Barnes, D.K.A.; Grange, L.J.; Morley, S.A. The extremes of disturbance gradients reduce functional redundancy of the shallow Antarctic benthos. Front. Mar. Sci. 2021, in press. [Google Scholar]
- Oliver, T. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Catilla, J.C.; Gattuso, J.-P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E.; et al. Rebuilding marine life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef]
- Peck, L.S. A cold limit to adapation in the sea. Trends Ecol. Evol. 2016, 31, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillinger, L.; Janussen, D.; Lundälv, T.; Richter, C. Rapid glass sponge expansion after climate-induced Antarctic ice shelf collapse. Cur. Biol. 2013, 23, 1330–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNFCC. Conference of the Parties, Adoption of the Paris Agreement. Doc FCCC/CP/2015/L.9/Rev.1. 2015. Available online: https://unfccc.int/sites/default/files/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 16 February 2022).
- Peck, L.S. Prospects for survival in the Southern Ocean: Vulnerability of benthic species to temperature change. Antarct. Sci. 2005, 17, 497–507. [Google Scholar] [CrossRef]
- Peck, L.S.; Brockington, S.; Vanhove, S.; Beghyn, M. Community recovery following catastrophic iceberg impacts in a soft-sediment shallow-water site at Signy Island, Antarctica. Mar. Ecol. Prog. Ser. 1999, 186, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dayton, P.K. Polar benthos. In Polar Oceanography; Smith, W.O., Ed.; Academic Press: London, UK, 1990; pp. 631–685. [Google Scholar]
- Mouillot, D.; Graham, N.A.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, S.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Griffiths, H.J.; Meijers, A.J.S.; Bracegirdle, T.J. More losers than winners in a century of Southern Ocean seafloor warming. Nat. Clim. Chang. 2017, 7, 749–754. [Google Scholar] [CrossRef]
- Morley, S.A.; Barnes, D.K.A.; Dunn, M.J. Predicting which species success in climate-forced polar seas. Front. Mar. Res. 2019, 5, 507. [Google Scholar] [CrossRef]
- Gutt, J. On the direct impact of ice on marine benthic communities, a review. Pol. Biol. 2001, 24, 553–564. [Google Scholar] [CrossRef]
- Smale, D.A.; Barnes, D.K.A.; Fraser, K.P.P. The influence of ice scour on benthic communities at three contrasting sites at Adelaide Island, Antarctica. Austral Ecol. 2007, 32, 878–888. [Google Scholar] [CrossRef]
- Deng, B. Fjords soak up a surprising amount of carbon. Nature 2015. [Google Scholar] [CrossRef]
- Manno, C.; Fielding, S.; Stowasser, G.; Murphy, E.J.; Tarling, G.A. Continuous moulting by Antarctic krill drives major pulses of carbon export in the north Scotia Sea, Southern Ocean. Nat. Com. 2020, 11, 6051. [Google Scholar] [CrossRef]
- Benayas, J.M.R.; Newton, A.C.; Diaz, A.; Bullock, J.M. Enhancement of Biodiversity and Ecosystem Services by Ecological Restoration: A Meta-Analysis. Science 2009, 325, 1121–1124. [Google Scholar] [CrossRef]
- Dodds, W.K.; Wilson, K.C.; Rehmeier, R.L.; Knight, G.L.; Wiggam, S.; Falke, J.A.; Dalgleish, H.J.; Bertrand, K.N. Comparing ecosystem goods and services provided by restored and native lands. BioScience 2008, 58, 837–845. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Harris, J.A. Restoration ecology: Repairing the Earth’s ecosystems in the new millennium. Rest. Ecol. 2001, 9, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Young, T.P. Restoration ecology and conservation biology. Biol. Conserv. 2000, 92, 73–83. [Google Scholar] [CrossRef] [Green Version]
- USEPA. Principles for the Ecological Restoration of Aquatic Resources; United States Environmental Protection Agency: Washington, DC, USA, 2000; 1p.
- Environment Canada How Much Habitat is Enough? Canadian Wildlife Service: Downsview, ON, Canada, 2004; 1p.
- Possingham, H.P.; Bode, M.; Klein, C.J. Optimal Conservation Outcomes Require Both Restoration and Protection. PLoS Biol. 2015, 13, e1002052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, A.; Jones, P.J.S. Protecting the ‘blue heart of the planet’: Strengthening the governance framework for marine protected areas beyond national jurisdiction. Mar. Policy 2021, 127, 104260. [Google Scholar] [CrossRef]
- Visalli, M.E.; Best, B.D.; Cabral, R.B.; William Cheung, W.W.L.; Clark, N.A.; Garilao, C.; Kaschner, K.; Kesner-Reyes, K.; Lam, V.W.Y.; Maxwell, S.M.; et al. Data-driven approach for highlighting priority areas for protection in marine areas beyond national jurisdiction. Mar. Policy 2020, 122, 103927. [Google Scholar] [CrossRef]
- Popova, E.; Vousden, D.; Sauer, W.H.H.; Mohammed, E.Y.; Allain, V.; Downey-Breedt, N.; Fletcher, R.; Gjerde, K.M.; Hal-pin, P.N.; Kelly, S.; et al. Ecological connectivity between the areas beyond national jurisdiction and coastal waters: Safeguarding interests of coastal communities in developing countries. Mar. Policy 2019, 104, 90–102. [Google Scholar] [CrossRef]






| Code | Functional Group |
|---|---|
| SP | pioneer sessile suspension |
| SC | climax sessile suspension |
| SS | sedentary suspension |
| SM | mobile suspension |
| DC | deposit feeding crawlers |
| DV | deposit feeding sedentary (soft) |
| DS | deposit feeding sedentary (hard/shelled) |
| GC | grazer |
| PS | scavenger/predator—sessile soft |
| PC | scavenger/predator—sessile hard/shelled |
| PM | scavenger/predator—mobile soft |
| PL | scavenger/predator—mobile hard/shelled |
| PA | scavenger/predator—arthropod |
| Flexible | flexible |
| Auto | autotroph |
| Parasite | parasitic |
| Organic C | Skeletal C | |||||
|---|---|---|---|---|---|---|
| Functional Group | 6 vs. 12 m | 6 vs. 20 m | 12 vs. 20 m | 6 vs. 12 m | 6 vs. 20 m | 12 vs. 20 m |
| PL | 21.7 | 19.7 | 12.9 | |||
| Flexible | 14.1 | 12.7 | 9.5 | 18.8 | 16.8 | 12.5 |
| PC | 11.9 | 13.2 | ||||
| DV | 10.1 | 14.3 | 14.8 | 12.9 | 12.5 | |
| SS | 16.2 | 18.3 | 16.2 | 18.3 | ||
| SP | 12.2 | 11.4 | 12.5 | |||
| PA | 12.7 |
| Organic C | Skeletal C | |||||
|---|---|---|---|---|---|---|
| Functional Group | Hangar vs. South | Hangar vs. Cheshire | South vs. Cheshire | Hangar vs. South | Hangar vs. Cheshire | South vs. Cheshire |
| SS | 33.8 | 14.7 | 11.3 | 32.1 | 11.0 | |
| GC | 12.7 | 15.4 | 11.8 | |||
| Flexible | 12.3 | 10.9 | ||||
| PL | 11.3 | 13.5 | 10.8 | 11.7 | 20.2 | 13.3 |
| SP | 12.6 | 15.0 | 11.7 | |||
| DV | 13.4 | 11.6 | ||||
| DS | 16.6 | |||||
| PA | 12.7 | 11.8 |
| Substratum | Standing Stock t C km−2 | Annual Productivity t C km−2 y−1 | Sequestered t C km−2 y−1 | |
|---|---|---|---|---|
| Benthic organic carbon | Hard | 648 ± 909 | 136 | 6.8 |
| Mixed | 504 ± 667 | 106 | 5.3 | |
| Soft | 391 ± 499 | 82 | 4.1 | |
| Macroalgae | Hard | 294 ± 181 | 179 | 10.7 |
| Mixed | 147 ± 90 | 89 | 5.4 | |
| Soft | - | - | - | |
| TOTAL | Hard | 942 | 315 | 17.5 |
| Mixed | 651 | 171 | 10.7 | |
| Soft | 391 | 82 | 4.1 |
| Substratum | Standing Stock t C | Productivity t C y−1 | Sequestered t C y−1 | Standing Stock t CO2e | Productivity t CO2e y−1 | Sequestered t CO2e y−1 |
|---|---|---|---|---|---|---|
| Hard | 179k | 60k | 3.3k | 656k | 294k | 12.2k |
| Mixed | 59k | 18k | 1.0k | 218k | 65k | 3.6k |
| Soft | 15k | 3k | 0.2k | 56k | 12k | 0.6k |
| TOTAL | 253k | 81k | 4.5k | 930k | 371k | 16.4k |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morley, S.A.; Souster, T.A.; Vause, B.J.; Gerrish, L.; Peck, L.S.; Barnes, D.K.A. Benthic Biodiversity, Carbon Storage and the Potential for Increasing Negative Feedbacks on Climate Change in Shallow Waters of the Antarctic Peninsula. Biology 2022, 11, 320. https://doi.org/10.3390/biology11020320
Morley SA, Souster TA, Vause BJ, Gerrish L, Peck LS, Barnes DKA. Benthic Biodiversity, Carbon Storage and the Potential for Increasing Negative Feedbacks on Climate Change in Shallow Waters of the Antarctic Peninsula. Biology. 2022; 11(2):320. https://doi.org/10.3390/biology11020320
Chicago/Turabian StyleMorley, Simon A., Terri A. Souster, Belinda J. Vause, Laura Gerrish, Lloyd S. Peck, and David K. A. Barnes. 2022. "Benthic Biodiversity, Carbon Storage and the Potential for Increasing Negative Feedbacks on Climate Change in Shallow Waters of the Antarctic Peninsula" Biology 11, no. 2: 320. https://doi.org/10.3390/biology11020320
APA StyleMorley, S. A., Souster, T. A., Vause, B. J., Gerrish, L., Peck, L. S., & Barnes, D. K. A. (2022). Benthic Biodiversity, Carbon Storage and the Potential for Increasing Negative Feedbacks on Climate Change in Shallow Waters of the Antarctic Peninsula. Biology, 11(2), 320. https://doi.org/10.3390/biology11020320