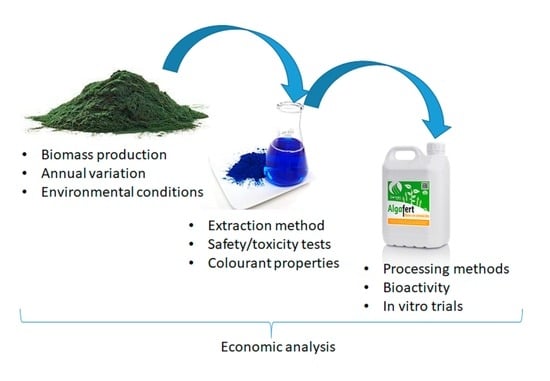
Biorefinery Approach Applied to the Production of Food Colourants and Biostimulants from Oscillatoria sp.
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Production
2.2. Extraction and Characterization of Phycocyanin
2.3. Colour Evaluation
2.4. Cytotoxicity
2.5. Biostimulant Capacity of the Waste Biomass
3. Results and Discussion
3.1. Microalgal Biomass Production
3.2. Phycocyanins Extraction
3.3. Utilization of the C-PC Extract as a Colourant
3.4. Stability of Coloured Beverages
3.5. Biostimulant Activity of the Residual Biomass
3.6. Techno-Economic Analysis of the Proposed Biorefinery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llamas, B.; Suárez-Rodríguez, M.C.; González-López, C.V.; Mora, P.; Acién, F.G. Techno-economic analysis of microalgae related processes for CO2 bio-fixation. Algal Res. 2021, 57, 102339. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Jespersen, L.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- MarketsandMarkets. Biostimulants Market by Active Ingredient, Crop Type, Application Method, Form—Global Forecast 2025. 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/biostimulant-market-1081.html (accessed on 27 July 2021).
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal Biofertilizers and Plant Growth Stimulants for Sustainable Agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in microalgae: A new opportunity for microalgal biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Bermejo, R. The application of a phycocyanin extract obtained from Arthrospira platensis as a blue natural colorant in beverages. J. Appl. Phycol. 2021, 33, 3059–3070. [Google Scholar] [CrossRef]
- Díaz, R.T.A.; Arrojo, V.C.; Agudo, M.A.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef]
- García, J.R.; Fernández, F.A.; Sevilla, J.F. Development of a process for the production of l-amino-acids concentrates from microalgae by enzymatic hydrolysis. Bioresour. Technol. 2012, 112, 164–170. [Google Scholar]
- Zucconi, F.; Forte, M.; Monaco, A.; De Bertoldi, M. Biological evaluation of compost maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Zhao, Z.R.; Wu, Z.L.; Huang, G.Q.; Li, G.R. An improved disk bioassay for determining activities of plant growth regulators. J. Plant Growth Regul. 1992, 11, 209–213. [Google Scholar] [CrossRef]
- Kuhnle, J.A.; Fuller, G.; Corse, J.; Mackey, B.E. Antisenescent Activity of Natural Cytokinins. Physiol. Plant. 1977, 41, 14–21. [Google Scholar] [CrossRef]
- Handler, R.M.; Canter, C.E.; Kalnes, T.N.; Lupton, F.S.; Kholiqov, O.; Shonnard, D.R.; Blowers, P. Evaluation of environmental impacts from microalgae cultivation in open-air raceway ponds: Analysis of the prior literature and investigation of wide variance in predicted impacts. Algal Res. 2012, 1, 83–92. [Google Scholar] [CrossRef]
- Rodríguez-Miranda, E.; Acién, F.G.; Guzmán, J.L.; Berenguel, M.; Visioli, A. A new model to analyze the temperature effect on the microalgae performance at large scale raceway reactors. Biotechnol. Bioeng. 2021, 118, 877–889. [Google Scholar] [CrossRef]
- Jain, A.; Behera, B.; Paramasivan, B. Evaluation of physicochemical procedures for pigment extraction from mixed microalgal consortium. Bioresour. Technol. Rep. 2021, 15, 100775. [Google Scholar] [CrossRef]
- Yap, B.H.; Dumsday, G.J.; Scales, P.J.; Martin, G.J. Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour. Technol. 2015, 184, 280–285. [Google Scholar] [CrossRef] [PubMed]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Kalil, S.J. Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef]
- de O Moreira, I.; Passos, T.S.; Chiapinni, C.; Silveira, G.K.; Souza, J.C.; Coca-Vellarde, L.G.; Deliza, R.; de Lima Araújo, K.G. Colour evaluation of a phycobiliprotein-rich extract obtained from Nostoc PCC9205 in acidic solutions and yogurt. J. Sci. Food Agric. 2011, 92, 598–605. [Google Scholar] [CrossRef]
- Mezquita, P.C.; Huerta, B.E.B.; Ramírez, J.C.P.; Hinojosa, C.P.O. Milks pigmentation with astaxanthin and determination of colour stability during short period cold storage. J. Food Sci. Technol. 2013, 52, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Rodríguez-Bermúdez, R.; Morillas-España, A.; Villaró, S.; García-Vaquero, M.; Morán, L.; Sánchez-Zurano, A.; González-López, C.V.; Acién-Fernández, F.G. Consumer knowledge and attitudes towards microalgae as food: The case of Spain. Algal Res. 2021, 54, 102174. [Google Scholar] [CrossRef]
- Hong, S.-I.; Han, J.H.; Krochta, J.M. Optical and surface properties of whey protein isolate coatings on plastic films as influenced by substrate, protein concentration, and plasticizer type. J. Appl. Polym. Sci. 2004, 92, 335–343. [Google Scholar] [CrossRef]
- Sonani, R.R.; Patel, S.; Bhastana, B.; Jakharia, K.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Purification and antioxidant activity of phycocyanin from Synechococcus sp. R42DM isolated from industrially polluted site. Bioresour. Technol. 2017, 245, 325–331. [Google Scholar] [CrossRef]
- Patel, S.N.; Sonani, R.R.; Jakharia, K.; Bhastana, B.; Patel, H.M.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Antioxidant activity and associated structural attributes of Halomicronema phycoerythrin. Int. J. Biol. Macromol. 2018, 111, 359–369. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef]
- Ördög, V.; Stirk, W.; Takács, G.; Pőthe, P.; Illés, A.; Bojtor, C.; Széles, A.; Tóth, B.; van Staden, J.; Nagy, J. Plant biostimulating effects of the cyanobacterium Nostoc piscinale on maize (Zea mays L.) in field experiments. S. Afr. J. Bot. 2021, 140, 153–160. [Google Scholar] [CrossRef]
- Ranglová, K.; Lakatos, G.E.; Câmara Manoel, J.A.; Grivalský, T.; Suárez Estrella, F.; Acién Fernández, F.G.; Molnár, Z.; Ördög, V.; Masojídek, J. Growth, biostimulant and biopesticide activity of the MACC-1 Chlorella strain cultivated outdoors in inorganic medium and wastewater. Algal Res. 2021, 53, 102136. [Google Scholar]
- Stirk, W.; Bálint, P.; Tarkowská, D.; Novak, O.; Strnad, M.; Ördög, V.; van Staden, J. Hormone profiles in microalgae: Gibberellins and brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef] [PubMed]







| Method | pH | FI (mm) | C-PC (mg/mL) | Purity Ratio (Amax/A280) | C-PC Extracted (% w/w) |
|---|---|---|---|---|---|
| Direct (5 min stirring) | 6.5 | 5 | 0.23 | 0.146 | 0.33 |
| Freeze–thawing | 6.5 | 5 | 0.18 | 0.100 | 0.26 |
| Freeze–thawing | 6.5 | 50 | 0.34 | 0.150 | 0.37 |
| Freeze–thawing | 6.5 | 100 | 0.42 | 0.190 | 0.45 |
| Freeze–thawing | 6.5 | 175 | 0.43 | 0.180 | 0.46 |
| Freeze–thawing | 6.5 | 250 | 0.30 | 0.160 | 0.49 |
| US + Freeze–thawing | 7.0 | 100 | 0.34 | 0.182 | 0.49 |
| US + Freeze–thawing | 6.5 | 100 | 0.34 | 0.187 | 0.48 |
| US + Freeze–thawing | 5.5 | 100 | 0.33 | 0.180 | 0.47 |
| US + Freeze–thawing | 5.0 | 100 | 0.33 | 0.170 | 0.46 |
| US + Stirring 45 min + Freeze–thawing | 6.5 | 100 | 0.32 | 0.173 | 0.50 |
| Product Type | Commercial Brand | Extract Volume Added (μL) | ∆E*ab Reached | Staining Factor (mg·L−1) |
|---|---|---|---|---|
| Isotonic Drinks | A | 610 | 7.92 | 86.4 |
| B | 750 | 8.06 | 105.8 | |
| C | 650 | 7.94 | 92.3 | |
| Tonic Drink | D | 150 | 1.41 | 22.0 |
| Wine | E | 130 | 5.62 | 47.9 |
| Base Case | |
|---|---|
| Production scale, ha | 1.00 |
| Biomass productivity, t/ha·year | 52.00 |
| C-PC content, % d.wt. | 0.50 |
| Biostimulant ratio, L/kg | 5.00 |
| Biomass production, kg/year | 52,000.00 |
| Biomass production cost, €/kg | 5.00 |
| Biomass production cost, €/year | 260,000.00 |
| C-PC production, kg/year | 260.00 |
| C-PC extraction cost, €/kg | 30.00 |
| C-PC extraction cost, €/year | 7800.00 |
| Biostimulant production, kg/year | 260,000.00 |
| Biostimulant production cost, €/kg | 2.00 |
| Biostimulant production cost, €/year | 520,000.00 |
| Biomass value, €/kg | 10.00 |
| C-PC value, €/kg | 500.00 |
| Biostimulant value, €/kg | 5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morillas-España, A.; Bermejo, R.; Abdala-Díaz, R.; Ruiz, Á.; Lafarga, T.; Acién, G.; Fernández-Sevilla, J.M. Biorefinery Approach Applied to the Production of Food Colourants and Biostimulants from Oscillatoria sp. Biology 2022, 11, 1278. https://doi.org/10.3390/biology11091278
Morillas-España A, Bermejo R, Abdala-Díaz R, Ruiz Á, Lafarga T, Acién G, Fernández-Sevilla JM. Biorefinery Approach Applied to the Production of Food Colourants and Biostimulants from Oscillatoria sp. Biology. 2022; 11(9):1278. https://doi.org/10.3390/biology11091278
Chicago/Turabian StyleMorillas-España, Ainoa, Ruperto Bermejo, Roberto Abdala-Díaz, Ángela Ruiz, Tomás Lafarga, Gabriel Acién, and José María Fernández-Sevilla. 2022. "Biorefinery Approach Applied to the Production of Food Colourants and Biostimulants from Oscillatoria sp." Biology 11, no. 9: 1278. https://doi.org/10.3390/biology11091278
APA StyleMorillas-España, A., Bermejo, R., Abdala-Díaz, R., Ruiz, Á., Lafarga, T., Acién, G., & Fernández-Sevilla, J. M. (2022). Biorefinery Approach Applied to the Production of Food Colourants and Biostimulants from Oscillatoria sp. Biology, 11(9), 1278. https://doi.org/10.3390/biology11091278