Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
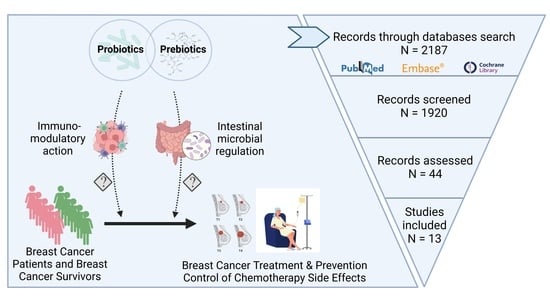
2.2. Literature Search, Study Selection, and Data Extraction
2.3. Risk of Bias Analysis
2.4. Subgroup Analysis
2.5. Statistics
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Subject Characteristics
3.4. Risk of Bias
3.5. Qualitative Analysis
3.6. Probiotics and Prebiotics
3.7. Body Mass Index
3.8. Percentage Change in Body Fat



3.9. Body Weight

3.10. Waist Circumference

3.11. Tumor Necrosis Factor-Alpha

3.12. High-Sensitivity C-Reactive Protein

3.13. Edema Volume

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mackowiak, P.A. Recycling metchnikoff: Probiotics, the intestinal microbiome and the quest for long life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef]
- WHO; FAO. Probiotics in Food: FAO Food and Nutrition Paper. 2006. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 3 March 2022).
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Marteau, P.R.; de Vrese, M.; Cellier, C.J.; Schrezenmeir, J. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 2001, 73 (Suppl. S2), 430s–436s. [Google Scholar] [CrossRef]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Hrdy, J.; Alard, J.; Couturier-Maillard, A.; Boulard, O.; Boutillier, D.; Delacre, M.; Lapadatescu, C.; Cesaro, A.; Blanc, P.; Pot, B.; et al. Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci. Rep. 2020, 10, 5345. [Google Scholar] [CrossRef]
- Zielinska, D.; Kolozyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. Biomed. Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef]
- Marteau, P.; Minekus, M.; Havenaar, R.; Huis in’t Veld, J.H. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: Validation and the effects of bile. J. Dairy Sci. 1997, 80, 1031–1037. [Google Scholar] [CrossRef]
- Pochart, P.; Marteau, P.; Bouhnik, Y.; Goderel, I.; Bourlioux, P.; Rambaud, J.C. Survival of bifidobacteria ingested via fermented milk during their passage through the human small intestine: An in vivo study using intestinal perfusion. Am. J. Clin. Nutr. 1992, 55, 78–80. [Google Scholar] [CrossRef]
- Gismondo, M.R.; Drago, L.; Lombardi, A. Review of probiotics available to modify gastrointestinal flora. Int. J. Antimicrob. Agents 1999, 12, 287–292. [Google Scholar] [CrossRef]
- Rafter, J.J. The role of lactic acid bacteria in colon cancer prevention. Scand. J. Gastroenterol. 1995, 30, 497–502. [Google Scholar] [CrossRef]
- Lu, K.; Dong, S.; Wu, X.; Jin, R.; Chen, H. Probiotics in Cancer. Front. Oncol. 2021, 11, 638148. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z.; et al. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224–1232. [Google Scholar] [CrossRef]
- McConnell, E.L.; Liu, F.; Basit, A.W. Colonic treatments and targets: Issues and opportunities. J. Drug Target. 2009, 17, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef]
- Aso, Y.; Akaza, H.; Kotake, T.; Tsukamoto, T.; Imai, K.; Naito, S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur. Urol. 1995, 27, 104–109. [Google Scholar] [CrossRef]
- Blaut, M. Relationship of prebiotics and food to intestinal microflora. Eur. J. Nutr. 2002, 41 (Suppl. S1), I11–I16. [Google Scholar] [CrossRef]
- Serban, D.E. Gastrointestinal cancers: Influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014, 345, 258–270. [Google Scholar] [CrossRef]
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Gao, Y.; Xue, Y.; Yan, Y.; Guo, X. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T.; et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Thol, K.; Pawlik, P.; McGranahan, N. Therapy sculpts the complex interplay between cancer and the immune system during tumour evolution. Genome Med. 2022, 14, 137. [Google Scholar] [CrossRef]
- Rodriguez-Arrastia, M.; Martinez-Ortigosa, A.; Rueda-Ruzafa, L.; Ayora, A.F.; Ropero-Padilla, C. Probiotic Supplements on Oncology Patients’ Treatment-Related Side Effects: A Systematic Review of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 4265. [Google Scholar] [CrossRef]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. Biomed. Pharmacother. 2020, 129, 110409. [Google Scholar]
- El-Nezami, H.S.; Polychronaki, N.N.; Ma, J.; Zhu, H.; Ling, W.; Salminen, E.K.; Juvonen, R.O.; Salminen, S.J.; Poussa, T.; Mykkänen, H.M. Probiotic supplementation reduces a biomarker for increased risk of liver cancer in young men from Southern China. Am. J. Clin. Nutr. 2006, 83, 1199–1203. [Google Scholar] [CrossRef]
- Goedert, J.J.; Jones, G.; Hua, X.; Xu, X.; Yu, G.; Flores, R.; Falk, R.T.; Gail, M.H.; Shi, J.; Ravel, J.; et al. Investigation of the association between the fecal microbiota and breast cancer in postmenopausal women: A population-based case-control pilot study. J. Natl. Cancer Inst. 2015, 107, djv147. [Google Scholar] [CrossRef]
- Su, S.J.; Yeh, T.M.; Chuang, W.J.; Ho, C.L.; Chang, K.L.; Cheng, H.L.; Liu, H.S.; Cheng, H.L.; Hsu, P.Y.; Chow, N.H. The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem. Pharmacol. 2005, 69, 307–318. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, A.S. Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J. Natl. Cancer Inst. 1998, 90, 381–388. [Google Scholar] [CrossRef]
- Molteni, A.; Brizio-Molteni, L.; Persky, V. In vitro hormonal effects of soybean isoflavones. J. Nutr. 1995, 125 (Suppl. S3), 751S–756S. [Google Scholar]
- Mendoza, L. Potential effect of probiotics in the treatment of breast cancer. Oncol. Rev. 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Van’t Veer, P.; Dekker, J.M.; Lamers, J.W.; Kok, F.J.; Schouten, E.G.; Brants, H.A.; Sturmans, F.; Hermus, R.J. Consumption of fermented milk products and breast cancer: A case-control study in The Netherlands. Cancer Res. 1989, 49, 4020–4023. [Google Scholar] [PubMed]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Br. Med. J. 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp Proc. 2006, 359, 63. [Google Scholar]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schunemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121 Pt 1, 1027–1031. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.J.A.C. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (Updated June 2017); Higgins, J.P.T., Churchill, R., Chandler, J.M.S.C., Eds.; Cochrane: Oxford, UK, 2017; Available online: https://training.cochrane.org/handbook (accessed on 3 March 2022).
- Institute of Medicine (U.S.) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research; Eden, J. Finding What Works in Health Care: Standards for Systematic Reviews; National Academies Press: Washington, DC, USA, 2011; p. xxii, 317.
- Nettleton, J.A.; Greany, K.A.; Thomas, W.; Wangen, K.E.; Adlercreutz, H.; Kurzer, M.S. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J. Nutr. 2004, 134, 1998–2003. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Greany, K.A.; Thomas, W.; Wangen, K.E.; Adlercreutz, H.; Kurzer, M.S. The effect of soy consumption on the urinary 2:16-hydroxyestrone ratio in postmenopausal women depends on equol production status but is not influenced by probiotic consumption. J. Nutr. 2005, 135, 603–608. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Greany, K.A.; Thomas, W.; Wangen, K.E.; Adlercreutz, H.; Kurzer, M.S. Short-term soy and probiotic supplementation does not markedly affect concentrations of reproductive hormones in postmenopausal women with and without histories of breast cancer. J. Altern. Complement. Med. 2005, 11, 1067–1074. [Google Scholar] [CrossRef]
- Donders, G.; Bellen, G.; Neven, P.; Grob, P.; Prasauskas, V.; Buchholz, S.; Ortmann, O. Effect of ultra-low-dose estriol and lactobacilli vaginal tablets (Gynoflor®) on inflammatory and infectious markers of the vaginal ecosystem in postmenopausal women with breast cancer on aromatase inhibitors. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Marschalek, J.; Farr, A.; Marschalek, M.L.; Domig, K.J.; Kneifel, W.; Singer, C.F.; Kiss, H.; Petricevic, L. Influence of Orally Administered Probiotic Lactobacillus Strains on Vaginal Microbiota in Women with Breast Cancer during Chemotherapy: A Randomized Placebo-Controlled Double-Blinded Pilot Study. Breast Care 2017, 12, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Vafa, S.; Zarrati, M.; Malakootinejad, M.; Totmaj, A.S.; Zayeri, F.; Salehi, M.; Sanati, V.; Haghighat, S. Calorie restriction and synbiotics effect on quality of life and edema reduction in breast cancer-related lymphedema, a clinical trial. Breast 2020, 54, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vafa, S.; Haghighat, S.; Janani, L.; Totmaj, A.S.; Navaei, M.; Amirinejad, A.; Emamat, H.; Salehi, Z.; Zarrati, M. The effects of synbiotic supplementation on serum inflammatory markers and edema volume in breast cancer survivors with lymphedema. EXCLI J. 2020, 19, 1–15. [Google Scholar]
- Totmaj, A.S.; Haghighat, S.; Jaberzadeh, S.; Navaei, M.; Vafa, S.; Janani, L.; Emamat, H.; Salehi, Z.; Izad, M.; Zarrati, M. The Effects of Synbiotic Supplementation on Serum Anti-Inflammatory Factors in the Survivors of Breast Cancer with Lymphedema following a Low Calorie Diet: A Randomized, Double-Blind, Clinical Trial. Nutr. Cancer 2022, 74, 869–881. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ippolito, M.; Monge, T.; Violi, R.; Cappello, P.; Ferrocino, I.; Cocolin, L.S.; De Francesco, A.; Bo, S.; Finocchiaro, C. Gut microbiota composition after diet and probiotics in overweight breast cancer survivors: A randomized open-label pilot intervention trial. Nutrition 2020, 74, 110749. [Google Scholar] [CrossRef]
- Lahiji, M.R.; Najafi, S.; Janani, L.; Yazdani, B.; Razmpoosh, E.; Zarrati, M. The effect of synbiotic on glycemic profile and sex hormones in overweight and obese breast cancer survivors following a weight-loss diet: A randomized, triple-blind, controlled trial. Clin. Nutr. 2021, 40, 394–403. [Google Scholar] [CrossRef]
- Lahiji, M.R.; Zarrati, M.; Najafi, S.; Yazdani, B.; Cheshmazar, E.; Razmpoosh, E.; Janani, L.; Raji Lahiji, M.; Shidfar, F. Effects of synbiotic supplementation on serum adiponectin and inflammation status of overweight and obese breast cancer survivors: A randomized, triple-blind, placebo-controlled trial. Support Care Cancer 2021, 29, 4147–4157. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2022, 161, 10–22. [Google Scholar] [CrossRef]
- Juan, Z.; Qing, Z.; Yongping, L.; Qian, L.; Wu, W.; Wen, Y.; Tong, J.; Ding, B. Probiotics for the Treatment of Docetaxel-Related Weight Gain of Breast Cancer Patients-A Single-Center, Randomized, Double-Blind, and Placebo-Controlled Trial. Front. Nutr. 2021, 8, 762929. [Google Scholar] [CrossRef]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatamleh, M.A.I.; Ahmad, S.; Boer, J.C.; Lim, J.K.; Chen, X.; Plebanski, M.; Mohamud, R. A perspective review on the role of nanomedicine in the modulation of TNF-TNFR2 axis in breast cancer immunotherapy. J. Oncol. 2019, 2019, 6313242. [Google Scholar] [CrossRef] [PubMed]
- Weitzenfeld, P.; Meron, N.; Leibovich-Rivkin, T.; Meshel, T.; Ben-Baruch, A. Progression of luminal breast tumors is promoted by a ménage à trois between the inflammatory cytokine TNFα and the hormonal and growth-supporting arms of the tumor microenvironment. Mediat. Inflamm. 2013, 2013, 720536. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, A.; Goettert, M.I.; de Souza, C.F.V. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Gupta, A.; Oyekunle, T.; Salako, O.; Daramola, A.; Alatise, O.; Ogun, G.; Adeniyi, A.; Deveaux, A.; Saraiya, V.; Hall, A.; et al. Association of high-sensitivity C-reactive protein and odds of breast cancer by molecular subtype: Analysis of the MEND study. Oncotarget 2021, 12, 1230–1242. [Google Scholar] [CrossRef]
- Norman, S.A.; Localio, A.R.; Potashnik, S.L.; Torpey, H.A.S.; Kallan, M.J.; Weber, A.L.; Weber, A.L.; Miller, L.T.; Demichele, A.; Solin, L.J. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J. Clin. Oncol. 2009, 27, 390–397. [Google Scholar] [CrossRef]
- DiSipio, T.; Rye, S.; Newman, B.; Hayes, S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Dayan, J.H.; Ly, C.L.; Kataru, R.P.; Mehrara, B.J. Lymphedema: Pathogenesis and Novel Therapies. Annu. Rev. Med. 2018, 69, 263–276. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Fotsis, T.; Heikkinen, R.; Dwyer, J.T.; Woods, M.; Goldin, B.R.; Gorbach, S.L. Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet 1982, 2, 1295–1299. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Sharma, D.; Jerome, G.; Santa-Maria, C.A. Obese breast cancer patients and survivors: Management considerations. Oncology 2018, 32, 410–417. [Google Scholar]
- Donders, G.; Neven, P.; Moegele, M.; Lintermans, A.; Bellen, G.; Prasauskas, V.; Grob, P.; Ortmann, O.; Buchholz, S. Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor(®)) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: Pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res. Treat. 2014, 145, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]


| Study Number | First Author, Year (Reference) | Country | Study Type | Participant Numbers (n) | Age Range (Years) | Probiotic Regimen | Dose | Duration |
|---|---|---|---|---|---|---|---|---|
| 1 | Nettleton, 2004 [41] | USA | Randomized crossover trial | 40 | 36–72 | ProLB + FOS | 3 capsules (109 CFU)/15–30 mg FOS before breakfast | 6 weeks |
| 2 | Nettleton, 2005a [42] | |||||||
| 3 | Nettleton, 2005b [43] | |||||||
| 4 | Donders, 2015 [44] | Belgium/Germany | Randomized trial | 16 | 52–63 | Lacto + ultra-low dose 0.03 mg estriol (E3) | 1 tablet (Gynoflor®) daily followed by maintenance therapy for 8 weeks | 4 weeks |
| 5 | Marschalek, 2017 [45] | Austria | Randomized placebo-controlled trial | 22 | 18–45 * | Lacto | 1 capsule (2.5 × 109 CFU) daily, twice/day | 2 weeks |
| 6 | Vafa, 2020 [46] | Iran | Parallel, randomized, placebo-controlled trial | 135 | 50–57 | ProLBS + FOS | 1 capsule (109 CFU)/38.5 mg FOS daily | 10 weeks |
| 7 | Vafa, 2022 [47] | Iran | Randomized clinical trial | 88 | 35–73 | ProLBS + FOS | 1 capsule (109 CFU)/38.5 mg FOS daily | 10 weeks |
| 8 | Totmaj, 2020 [48] | |||||||
| 9 | Pellegrini, 2020 [49] | Iran | Randomized open-label trial | 34 | <70 * | ProLB | 1 sachet (4 × 109 CFU) daily | 2 months |
| 10 | Lahiji, 2021a [50] | Iran | Randomized placebo-controlled | 76 | 50–75 | ProLBS + FOS | 1 capsule (109 CFU)/38.5 mg FOS daily | 8 weeks |
| 11 | Lahiji, 2021b [51] | |||||||
| 12 | Juan, 2022 [52] | China | Randomized placebo-controlled trial | 160 | 28–63 | ProLBE | 3 capsules (0.84 g) per time, twice/day | 3 weeks |
| 13 | Juan, 2021 [53] | 100 |
| Characteristics | Intervention | Placebo | Control |
|---|---|---|---|
| Total numbers, n (% total) | 290 (51%) | 218 (38%) | 63 (11%) |
| Age, mean (± SD) | 51.01 (8.78) | 51.33 (8.26) | 53.24 (3.55) |
| BMI (kg/m2), n (%) | |||
| < 25 | 96 (33%) | 80 (37%) | 0 |
| ≥ 25 | 183 (63%) | 127 (58%) | 63 (100%) |
| Unknown | 11 (4%) | 11 (5%) | 0 |
| Breast cancer stage, n (%) | |||
| Stage I | 44 (21%) | 19 (16%) | 27 (20%) |
| Stage II | 125 (59%) | 79 (66%) | 73 (55%) |
| Stage III | 44 (21%) | 21 (18%) | 32 (24%) |
| ER status, n (%) | |||
| Positive | 72 (76%) | 71 (76%) | 28 (74%) |
| Negative | 23 (24%) | 22 (24%) | 10 (26%) |
| PR status, n (%) | |||
| Positive | 70 (74%) | 59 (66%) | 28 (68%) |
| Negative | 25 (26%) | 30 (34%) | 13 (32%) |
| HER2 status, n (%) | |||
| Positive | 34 (19%) | 38 (22%) | 6 (32%) |
| Negative | 141 (81%) | 135 (78%) | 13 (68%) |
| Country, n (%) | |||
| USA | 40 (14%) | 0 | 0 |
| Belgium/Germany | 16 (6%) | 0 | 0 |
| Austria | 11 (4%) | 11 (5%) | 0 |
| Iran | 143 (49%) | 127 (58%) | 63 (100%) |
| China | 80 (28%) | 80 (37%) | 0 |
| # | Author, publication year (reference) | Population | Intervention | Control | Sample Type | Findings |
|---|---|---|---|---|---|---|
| 1 | Nettleton, 2004 [41] | Breast cancer (BC) survivors | 1. Diet+Soy protein isolate (S); 2. Diet+S+Probiotics (S+P) 3. Diet+Milk protein isolate (M); 4. Diet+M+Probiotics (M+P) *Four 42 d diet plan in random order | - | Plasma, 24 hr urine | 1. No changes in plasma phytoestrogen between groups. 2. No changes between S and S+P diets due to plasma phytoestrogen levels and number of equol producers. 3. Probiotic supplement does not generally affect plasma isoflavones. |
| 2 | Nettleton, 2005a [42] | 1. Soy consumption tended to increase urinary 2-OHE (p = 0.07) and 16α-OHE1 (p = 0.11) but had no effect on urinary 2:16OHE1. 2. Soy consumption increased 2:16OHE1 only in women who are equol producers. | ||||
| 3 | Nettleton, 2005b [43] | 1. Soy, probiotic supplements, or equol producer status had no impact on hormone levels. 2. Neither presence of cancernor or equol producers changed the effects of soy or probiotics. | ||||
| 4 | Donders, 2015 [44] | Postmenopausal BC survivors on aromatase inhibitors with severe atrophic vaginitis | Vaginal use of 0.03 mg estriol and lactobacilli (1 tablet of Gynoflor® for 28 d) combination | - | Vaginal smear | 1. Lactobacillary grades (p < 0.001) and aerobic vaginitis (p < 0.01) improved during treatment. 2. Leukocytes (p < 0.01) and parabasal cells (Ptrend < 0.01) dropped at the final visit. 3. Candida may develop soon after its use but rapidly disappears again upon their prolonged use. |
| 5 | Marschalek, 2017 [45] | Postmenopausal BC patients receiving chemotherapy, with vaginal atrophy and an intermediate vaginal microbiota (Nugent score 4–6) | Twice daily oral capsules for 2 weeks | Oral placebo having lactose | Vaginal smear | 1. Observed a positive influence on vaginal microbiota in 63% women in the intervention group and 36% women in the control group. 2. There was a shift in Nugent score towards normal microbiota levels in the intervention group and significant deterioration in the score in the control group. |
| 6 | Vafa, 2020 [46] | BC survivors with breast-cancer-related lymphedema (BCRL) | A calorie-restricted diet plus a synbiotic (CRS) daily for 10 weeks | Diet plus a placebo (CRP) and control | Body fluid | 1. A decrease in the total quality-of-life score (p = 0.004), and its psychosocial (p = 0.022) and functional (p = 0.002) domain scores 2. A decrease in edema volume (p = 0.002) and BMI (p < 0.001) in comparison to controls. |
| 7 | Vafa, 2022 [47] | Overweight or obese BC survivors with BCRL | Low-calorie diet (LCD) plus a synbiotic daily for 10 weeks | LCD plus a placebo | Serum | 1. Had beneficial effects on increasing serum TGF-β, IL-10, and adiponectin levels in women with BCRL, but no significant differences. 2. Edema volume decreased in the synbiotic group. 3. BW, BMI, BF%, and WC decreased in both groups. |
| 8 | Totmaj, 2020 [48] | 1. A significant reduction in leptin (p = 0.003) and TNF-α (p = 0.039) between the groups. 2. No significant effects in hs-CRP (p = 0.55) and IL-1β (p = 0.118) between study groups. | ||||
| 9 | Pellegrini, 2020 [49] | Overweight BC survivors | Mediterranean diet for 4 mo. + Probiotics for first 2 mo. | Mediterranean diet for 4 mo. only | Serum, stool | 1. Number of bacterial spp. (p = 0.01) and diversity (p = 0.004) significantly increased only with intervention. 2. Bacteroidetes:Firmicutes ratio decreased with intervention and increased in controls (p = 0.004). 3. Significant improvement in metabolic and anthropometric parameters (BW, BMI, glucose, and insulin) compared with Mediterranean diet alone |
| 10 | Lahiji, 2021a [50] | Overweight or obese postmenopausal BC survivors | LCD + 109 CFU/day of synbiotics for 8 weeks | LCD + Placebo | Serum | 1. Insignificant reducing effects on glycemic profile (serum insulin, fasting plasma glucose, HbA1c, HOMA-IR), IGF-1, and sex hormones (estradiol, testosterone, DHEA-S, and SHBG). |
| 11 | Lahiji, 2021b [51] | 1. Increased adiponectin (p < 0.001), reduced TNF-α (p < 0.001) and hs-CRP (p < 0.001) compared to placebo. | ||||
| 12 | Juan, 2022 [52] | BC patients who underwent 4 cycles of docetaxel-based chemotherapy | Twice daily, 3 capsules (0.84 g)/time of probiotics during chemotherapy at a cycle of 21 d for a total of four cycles | Placebo | Plasma, stool | 1. Supplement significantly decreased the CRCI, improved the allover cognitive functions, changed gut microbial, and modulated 9 plasma metabolite changes. 2. Metabolites p-mentha-1,8-dien-7-ol, linoelaidyl carnitine, and 1-aminocyclopropane-1-carboxylic acid negatively correlated with rate of CRCI. |
| 13 | Juan, 2021 [53] | 1. Bacteroides (p < 0.001) and Anaerostipes (p < 0.001) changes inversely correlated with change in LDL. 2. Reduced BW, BF%, and LDL, and minimized metabolic changes and gut dysbacteriosis. |
| Subgroup/Sensitivity Analysis | Number of Trials | SMD (95% CI) | p-Value | Heterogeneity (I2, p-Value) | |
|---|---|---|---|---|---|
| BMI | |||||
| Probiotics ± prebiotics | Probiotics only | 2 | 0.00 (−0.76, 0.77) | 0.99 | 73% (0.05) |
| Combined with FOS | 3 | −0.05 (−0.29, 0.20) | 0.72 | 0% (0.99) | |
| Intake duration | 10 weeks | 3 | −0.06 (−0.30, 0.19) | 0.65 | 0% (1.00) |
| 8 weeks | 2 | 0.14 (−0.30, 0.58) | 0.53 | 19% (0.27) | |
| 3 weeks | 1 | −0.34 (−0.75, 0.07) | 0.11 | N/A | |
| Body weight | |||||
| Probiotics ± prebiotics | Probiotics only | 2 | 0.10 (−1.08, 1.28) | 0.87 | 88% (0.004) |
| Combined with FOS | 2 | −0.01 (−0.32, 0.30) | 0.93 | 0% (0.54) | |
| Intake duration | 10 weeks | 1 | 0.08 (−0.34, 0.49) | 0.73 | N/A |
| 8 weeks | 2 | 0.27 (−0.57, 1.10) | 0.53 | 75% (0.04) | |
| 3 weeks | 1 | −0.47 (−0.88, −0.06) | 0.03 | N/A | |
| BF% | |||||
| Probiotics ± prebiotics | Probiotics only | 1 | −4.5 (−5.28, −3.72) | <0.00001 | N/A |
| Combined with FOS | 2 | −0.03 (−0.34, 0.28) | 0.85 | 0% (0.86) | |
| Intake duration | 10 weeks | 1 | −0.00 (−0.42, 0.41) | 0.98 | N/A |
| 8 weeks | 1 | −0.06 (−0.52, 0.40) | 0.80 | N/A | |
| 3 weeks | 1 | −4.50 (−5.28, −3.72) | <0.00001 | N/A | |
| Waist circumference | |||||
| Probiotics ± prebiotics | Probiotics only | 1 | 4.0 (−1.44, 9.44) | 0.15 | N/A |
| Combined with FOS | 2 | −1.10 (−4.52, 2.31) | 0.53 | 0% (0.84) | |
| Intake duration | 10 weeks | 1 | −0.14 (−0.56, 0.28) | 0.36 | 0% (1.00) |
| 8 weeks | 2 | 0.19 (−0.24, 0.63) | 0.39 | 18% (0.27) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thu, M.S.; Ondee, T.; Nopsopon, T.; Farzana, I.A.K.; Fothergill, J.L.; Hirankarn, N.; Campbell, B.J.; Pongpirul, K. Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis. Biology 2023, 12, 280. https://doi.org/10.3390/biology12020280
Thu MS, Ondee T, Nopsopon T, Farzana IAK, Fothergill JL, Hirankarn N, Campbell BJ, Pongpirul K. Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis. Biology. 2023; 12(2):280. https://doi.org/10.3390/biology12020280
Chicago/Turabian StyleThu, May S., Thunnicha Ondee, Tanawin Nopsopon, Izzati A. K. Farzana, Joanne L. Fothergill, Nattiya Hirankarn, Barry J. Campbell, and Krit Pongpirul. 2023. "Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis" Biology 12, no. 2: 280. https://doi.org/10.3390/biology12020280
APA StyleThu, M. S., Ondee, T., Nopsopon, T., Farzana, I. A. K., Fothergill, J. L., Hirankarn, N., Campbell, B. J., & Pongpirul, K. (2023). Effect of Probiotics in Breast Cancer: A Systematic Review and Meta-Analysis. Biology, 12(2), 280. https://doi.org/10.3390/biology12020280