Chasing the Role of miRNAs in RCC: From Free-Circulating to Extracellular-Vesicle-Derived Biomarkers
Abstract
:Simple Summary
Abstract
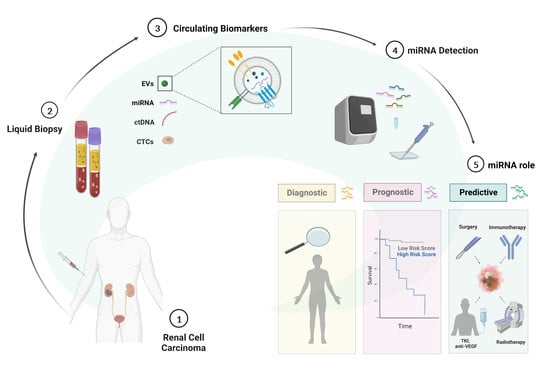
1. Renal Cell Carcinoma: Clinical Diagnosis and Staging
2. RCC Treatments: Advances and Challenges in the Lack of Biomarkers
3. Liquid Biopsy in RCC
4. Extracellular Vesicles: Biogenesis and Role in RCC
5. MicroRNAs (miRNAs): From Free-Circulating to EV-Packaged Biomarkers
6. The Potential Role of Blood-Circulating miRNAs in RCC
| miRNA | Expression Changes in RCC | Source | Therapeutic Role | Reference |
|---|---|---|---|---|
| miR-106 | Upregulated | Serum | Diagnostic and predictive | Tusong et al., 2017 [58] |
| miR-122-5p | Upregulated | Serum | Prognostic | Heinemann et al., 2018 [79] |
| miR-1233 | Upregulated | Exosomes— serum | Diagnostic and predictive | Zhang et al., 2018 [67] |
| Upregulated | Plasma | Diagnostic and prognostic | Dias et al., 2017 [63] | |
| Upregulated | Serum | Diagnostic | Wulfken et al., 2011 [66] | |
| miR-141 | Downregulated | Serum | Diagnostic | Cheng et al., 2013 [59] |
| miR-144-3p | Upregulated | Plasma | Diagnostic and prognostic | Lou et al., 2017 [76] |
| miR-149-3p | Upregulated | Exosomes— plasma | Diagnostic | Xiao et al., 2020 [65] |
| miR-150 | Downregulated | Plasma | Prognostic | Chanudet et al., 2017 [80] |
| miR-182-5p | Downregulated | Serum | Diagnostic | Huang et al., 2020 [68] |
| miR-187 | Downregulated | Plasma | Diagnostic and prognostic | Zhao et al., 2013 [77] |
| miR-193a-3p | Upregulated | Serum | Diagnostic | Wang et al., 2015 [70] |
| miR-196a | Upregulated | Serum | Diagnostic and prognostic | Huang et al., 2020 [75] |
| miR-206 | Upregulated | Serum | Prognostic | Heinemann et al., 2018 [79] |
| miR-20b-5p | Downregulated | Serum | Diagnostic | Huang et al., 2020 [75] |
| miR-21 | Upregulated | Serum | Diagnostic and predictive | Tusong et al., 2017 [58] |
| Upregulated | Serum | Diagnostic and prognostic | Cheng et al., 2013 [59] | |
| Upregulated | Serum | Diagnostic and prognostic | Liu et al., 2017 [60] | |
| miR-210 | Upregulated | Serum | Diagnostic and predictive | Fedorko et al., 2015 [71] |
| Upregulated | Exosomes— serum | Diagnostic and predictive | Zhang et al., 2018 671] | |
| Upregulated | Plasma | Diagnostic and prognostic | Dias et al., 2017 [63] | |
| Upregulated | Serum | Diagnostic | Iwamoto et al., 2014 [73] | |
| Upregulated | Serum | Diagnostic and predictive | Zhao et al., 2013 [72] | |
| miR-218 | Upregulated | Plasma | Diagnostic | Dias et al., 2017 [63] |
| miR-22 | Downregulated | Serum | Diagnostic, prognostic, and predictive | Li et al., 2017 [84] |
| miR-221 | Upregulated | Plasma | Diagnostic and prognostic | Teixeira et al., 2014 [64] |
| Upregulated | Plasma | Diagnostic and prognostic | Dias et al., 2017 [63] | |
| miR-222 | Upregulated | Plasma | Diagnostic | Teixeira et al., 2014 [64] |
| miR-224 | Upregulated | Serum | Diagnostic | Cheng et al., 2013 [59] |
| Upregulated | Serum | Diagnostic | Huang et al., 2020 [68] | |
| miR-28-5p | Downregulated | Serum | Diagnostic | Wang et al., 2015 [70] |
| miR-30a-5p | Downregulated | Serum | Diagnostic | Huang et al., 2020 [75] |
| miR-34a | Upregulated | Serum | Diagnostic | Cheng et al., 2013 [59] |
| miR-34b-3p | Downregulated | Serum | Diagnostic | Huang et al., 2020 [68] |
| miR-362 | Upregulated | Serum | Diagnostic | Wang et al., 2015 [70] |
| miR-378 | Upregulated | Serum | Diagnostic and predictive | Fedorko et al., 2015 [71] |
| Downregulated | Serum | Diagnostic | Wang et al., 2015 [70] | |
| Upregulated | Serum | Diagnostic | Redova et al., 2012 [69] | |
| miR-424-3p | Upregulated | Exosomes— plasma | Diagnostic | Xiao et al., 2020 [65] |
| miR-451 | Downregulated | Serum | Diagnostic | Redova et al., 2012 [69] |
| miR-483-5p | Downregulated | Plasma | Diagnostic, prognostic, and predictive | Wang et al., 2021 [83] |
| miR-508-3p | Downregulated | Serum | Diagnostic | Liu et al., 2019 [61] |
| miR-572 | Upregulated | Serum | Diagnostic | Wang et al., 2015 [70] |
| miR-625-3p | Downregulated | Serum | Diagnostic | Zhao et al., 2019 [62] |
| miR-765 | Downregulated | Plasma | Predictive | Xiao et al., 2020 [74] |
| miR-885-5p | Upregulated | Serum | Diagnostic | Liu et al., 2019 [61] |
| miR-92a-1-5p | Downregulated | Exosomes— plasma | Diagnostic | Xiao et al., 2020 [65] |
| miR-1293 | Downregulated | EVs— plasma | Prognostic and predictive | Dias et al., 2020 [82] |
| miR-301a-3p | Upregulated | EVs— plasma | Prognostic and predictive | Dias et al., 2020 [82] |
| miR-let-7i-5p | Downregulated | Exosomes— plasma | Prognostic | Du et al., 2017 [81] |
| miR-183-5p | Upregulated | Serum | Diagnostic and prognostic | Zhang et al., 2015 [78] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, L.; Wang, M.; Li, H.; You, P. MicroRNAs in Body Fluids: A More Promising Biomarker for Clear Cell Renal Cell Carcinoma. Cancer Manag. Res. 2021, 13, 7663–7675. [Google Scholar] [CrossRef]
- Spadaccino, F.; Gigante, M.; Netti, G.S.; Rocchetti, M.T.; Franzin, R.; Gesualdo, L.; Castellano, G.; Stallone, G.; Ranieri, E. The Ambivalent Role of MiRNAs in Carcinogenesis: Involvement in Renal Cell Carcinoma and Their Clinical Applications. Pharmaceuticals 2021, 14, 322. [Google Scholar] [CrossRef]
- Singh, D. Current Updates and Future Perspectives on the Management of Renal Cell Carcinoma. Life Sci. 2021, 264, 118632. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Yang, C.; Liao, Z. Comparison of Radical Nephrectomy and Partial Nephrectomy for T1 Renal Cell Carcinoma: A Meta-Analysis. Urol. Int. 2018, 101, 175–183. [Google Scholar] [CrossRef]
- Alam, R.; Patel, H.D.; Osumah, T.; Srivastava, A.; Gorin, M.A.; Johnson, M.H.; Trock, B.J.; Chang, P.; Wagner, A.A.; McKiernan, J.M.; et al. Comparative Effectiveness of Management Options for Patients with Small Renal Masses: A Prospective Cohort Study. BJU Int. 2019, 123, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Novick, A.C.; Belldegrun, A.; Blute, M.L.; Chow, G.K.; Derweesh, I.H.; Faraday, M.M.; Kaouk, J.H.; Leveillee, R.J.; Matin, S.F.; et al. Guideline for Management of the Clinical T1 Renal Mass. J. Urol. 2009, 182, 1271–1279. [Google Scholar] [CrossRef]
- Marchioni, M.; Rivas, J.G.; Autran, A.; Socarras, M.; Albisinni, S.; Ferro, M.; Schips, L.; Scarpa, R.M.; Papalia, R.; Esperto, F. Biomarkers for Renal Cell Carcinoma Recurrence: State of the Art. Curr. Urol. Rep. 2021, 22, 31. [Google Scholar] [CrossRef] [PubMed]
- Borchiellini, D.; Maillet, D. Clinical Activity of Immunotherapy-Based Combination First-Line Therapies for Metastatic Renal Cell Carcinoma: The Right Treatment for the Right Patient. Bull. Cancer 2022, 109, 2S4–2S18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Rini, B.I.; Beckermann, K.E. Emerging Targets in Clear Cell Renal Cell Carcinoma. Cancers 2022, 14, 4843. [Google Scholar] [CrossRef]
- Tung, I.; Sahu, A. Immune Checkpoint Inhibitor in First-Line Treatment of Metastatic Renal Cell Carcinoma: A Review of Current Evidence and Future Directions. Front. Oncol. 2021, 11, 707214. [Google Scholar] [CrossRef] [PubMed]
- Iaxx, R.; Lefort, F.; Domblides, C.; Ravaud, A.; Bernhard, J.-C.; Gross-Goupil, M. An Evaluation of Cabozantinib for the Treatment of Renal Cell Carcinoma: Focus on Patient Selection and Perspectives. Ther. Clin. Risk Manag. 2022, 18, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Nolè, F.; Verri, E.; Renne, G.; Paglino, C.; Santoni, M.; Cossu Rocca, M.; Giglione, P.; Aurilio, G.; Cullurà, D.; et al. Prognostic Role of PD-L1 Expression in Renal Cell Carcinoma. A Systematic Review and Meta-Analysis. Target. Oncol. 2016, 11, 143–148. [Google Scholar] [CrossRef]
- Carlsson, J.; Sundqvist, P.; Kosuta, V.; Fält, A.; Giunchi, F.; Fiorentino, M.; Davidsson, S. PD-L1 Expression Is Associated With Poor Prognosis in Renal Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 213. [Google Scholar] [CrossRef]
- Dudani, S.; Savard, M.-F.; Heng, D.Y.C. An Update on Predictive Biomarkers in Metastatic Renal Cell Carcinoma. Eur. Urol. Focus 2020, 6, 34–36. [Google Scholar] [CrossRef]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to systemic therapies in clear cell renal cell carcinoma: Mechanisms and management strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grange, C.; Brossa, A.; Bussolati, B. Extracellular Vesicles and Carried MiRNAs in the Progression of Renal Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Martins, V.R.; Dias, M.S.; Hainaut, P. Tumor-Cell-Derived Microvesicles as Carriers of Molecular Information in Cancer. Curr. Opin. Oncol. 2013, 25, 66–75. [Google Scholar] [CrossRef]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-Based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, H.; Rutishauser, D.; Schraml, P.; Moch, H.; Bolck, H.A. Liquid Biopsies in Renal Cell Carcinoma—Recent Advances and Promising New Technologies for the Early Detection of Metastatic Disease. Front. Oncol. 2020, 10, 582843. [Google Scholar] [CrossRef] [PubMed]
- Michela, B. Liquid Biopsy: A Family of Possible Diagnostic Tools. Diagnostics 2021, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, L.; Zheng, J.; Li, Z.; Li, S.; Wang, K.; Chen, X. Liquid Biopsy at the Frontier in Renal Cell Carcinoma: Recent Analysis of Techniques and Clinical Application. Mol. Cancer 2023, 22, 37. [Google Scholar] [CrossRef]
- Bade, R.M.; Schehr, J.L.; Emamekhoo, H.; Gibbs, B.K.; Rodems, T.S.; Mannino, M.C.; Desotelle, J.A.; Heninger, E.; Stahlfeld, C.N.; Sperger, J.M.; et al. Development and Initial Clinical Testing of a Multiplexed Circulating Tumor Cell Assay in Patients with Clear Cell Renal Cell Carcinoma. Mol. Oncol. 2021, 15, 2330–2344. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Berchuck, J.E.; Korthauer, K.; Spisak, S.; Nassar, A.H.; Abou Alaiwi, S.; Chakravarthy, A.; Shen, S.Y.; Bakouny, Z.; Boccardo, F.; et al. Detection of Renal Cell Carcinoma Using Plasma and Urine Cell-Free DNA Methylomes. Nat. Med. 2020, 26, 1041–1043. [Google Scholar] [CrossRef]
- Sequeira, J.P.; Constâncio, V.; Salta, S.; Lobo, J.; Barros-Silva, D.; Carvalho-Maia, C.; Rodrigues, J.; Braga, I.; Henrique, R.; Jerónimo, C. LiKidMiRs: A DdPCR-Based Panel of 4 Circulating MiRNAs for Detection of Renal Cell Carcinoma. Cancers 2022, 14, 858. [Google Scholar] [CrossRef]
- Peter, M.R.; Zhao, F.; Jeyapala, R.; Kamdar, S.; Xu, W.; Hawkins, C.; Evans, A.J.; Fleshner, N.E.; Finelli, A.; Bapat, B. Investigating Urinary Circular RNA Biomarkers for Improved Detection of Renal Cell Carcinoma. Front. Oncol. 2021, 11, 814228. [Google Scholar] [CrossRef]
- Kohli, M.; Tan, W.; Vire, B.; Liaud, P.; Blairvacq, M.; Berthier, F.; Rouison, D.; Garnier, G.; Payen, L.; Cousin, T.; et al. Prognostic Value of Plasma HPG80 (Circulating Progastrin) in Metastatic Renal Cell Carcinoma. Cancers 2021, 13, 375. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Zhang, P.; Li, H.-C.; Yang, X.-J.; Zhang, Y.-P.; Li, Z.-L.; Xue, L.; Xue, Y.-Q.; Li, H.-L.; Chen, Q.; et al. Dynamic Changes of Different Phenotypic and Genetic Circulating Tumor Cells as a Biomarker for Evaluating the Prognosis of RCC. Cancer Biol. Ther. 2019, 20, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Uemura, M.; Fujita, M.; Maejima, K.; Koh, Y.; Matsushita, M.; Nakano, K.; Hayashi, Y.; Wang, C.; Ishizuya, Y.; et al. Clinical Significance of the Mutational Landscape and Fragmentation of Circulating Tumor DNA in Renal Cell Carcinoma. Cancer Sci. 2019, 110, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Shen, Q.; Yang, X.; Qiu, Y.; Zhang, W. The Role of Extracellular Vesicles: An Epigenetic View of the Cancer Microenvironment. BioMed Res. Int. 2015, 2015, 649161. [Google Scholar] [CrossRef] [Green Version]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [Green Version]
- Lindoso, R.S.; Collino, F.; Camussi, G. Extracellular Vesicles Derived from Renal Cancer Stem Cells Induce a Pro-Tumorigenic Phenotype in Mesenchymal Stromal Cells. Oncotarget 2015, 6, 7959–7969. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Rimmer, M.P.; Gregory, C.D.; Mitchell, R.T. Extracellular Vesicles in Urological Malignancies. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2021, 1876, 188570. [Google Scholar] [CrossRef]
- Barth, D.A.; Drula, R.; Ott, L.; Fabris, L.; Slaby, O.; Calin, G.A.; Pichler, M. Circulating Non-Coding RNAs in Renal Cell Carcinoma—Pathogenesis and Potential Implications as Clinical Biomarkers. Front. Cell Dev. Biol. 2020, 8, 828. [Google Scholar] [CrossRef]
- Butz, H.; Nofech-Mozes, R.; Ding, Q.; Khella, H.W.Z.; Szabó, P.M.; Jewett, M.; Finelli, A.; Lee, J.; Ordon, M.; Stewart, R.; et al. Exosomal MicroRNAs Are Diagnostic Biomarkers and Can Mediate Cell–Cell Communication in Renal Cell Carcinoma. Eur. Urol. Focus 2016, 2, 210–218. [Google Scholar] [CrossRef]
- Horie, K.; Kawakami, K.; Fujita, Y.; Sugaya, M.; Kameyama, K.; Mizutani, K.; Deguchi, T.; Ito, M. Exosomes Expressing Carbonic Anhydrase 9 Promote Angiogenesis. Biochem. Biophys. Res. Commun. 2017, 492, 356–361. [Google Scholar] [CrossRef]
- Jingushi, K.; Uemura, M.; Ohnishi, N.; Nakata, W.; Fujita, K.; Naito, T.; Fujii, R.; Saichi, N.; Nonomura, N.; Tsujikawa, K.; et al. Extracellular Vesicles Isolated from Human Renal Cell Carcinoma Tissues Disrupt Vascular Endothelial Cell Morphology via Azurocidin. Int. J. Cancer 2018, 142, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Grange, C.; Tapparo, M.; Tritta, S.; Deregibus, M.C.; Battaglia, A.; Gontero, P.; Frea, B.; Camussi, G. Role of HLA-G and Extracellular Vesicles in Renal Cancer Stem Cell-Induced Inhibition of Dendritic Cell Differentiation. BMC Cancer 2015, 15, 1009. [Google Scholar] [CrossRef] [Green Version]
- Jansson, M.D.; Lund, A.H. MicroRNA and Cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-Mediated Transfer of MicroRNAs within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellinger, J.; Gevensleben, H.; Müller, S.C.; Dietrich, D. The Emerging Role of Non-Coding Circulating RNA as a Biomarker in Renal Cell Carcinoma. Expert Rev. Mol. Diagn. 2016, 16, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Shen, Y.; Chen, Z.; Li, R.; Ge, Q. No Significant Difference between Plasma MiRNAs and Plasma-Derived Exosomal MiRNAs from Healthy People. BioMed Res. Int. 2017, 2017, e1304816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhome, R.; Del Vecchio, F.; Lee, G.-H.; Bullock, M.D.; Primrose, J.N.; Sayan, A.E.; Mirnezami, A.H. Exosomal MicroRNAs (ExomiRs): Small Molecules with a Big Role in Cancer. Cancer Lett. 2018, 420, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Cinque, A.; Vago, R.; Trevisani, F. Circulating RNA in Kidney Cancer: What We Know and What We Still Suppose. Genes 2021, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Juracek, J.; Madrzyk, M.; Stanik, M.; Slaby, O. Urinary MicroRNAs and Their Significance in Prostate Cancer Diagnosis: A 5-Year Update. Cancers 2022, 14, 3157. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Padmanabhan, G. MiRNAs as Potential Biomarker of Kidney Diseases: A Review. Cell Biochem. Funct. 2020, 38, 990–1005. [Google Scholar] [CrossRef]
- Lokeshwar, S.D.; Talukder, A.; Yates, T.J.; Hennig, M.J.P.; Garcia-Roig, M.; Lahorewala, S.S.; Mullani, N.N.; Klaassen, Z.; Kava, B.R.; Manoharan, M.; et al. Molecular Characterization of Renal Cell Carcinoma: A Potential Three-MicroRNA Prognostic Signature. Cancer Epidemiol. Biomark. Prev. 2018, 27, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Tsiakanikas, P.; Giaginis, C.; Kontos, C.K.; Scorilas, A. Clinical Utility of MicroRNAs in Renal Cell Carcinoma: Current Evidence and Future Perspectives. Expert Rev. Mol. Diagn. 2018, 18, 981–991. [Google Scholar] [CrossRef]
- Tusong, H.; Maolakuerban, N.; Guan, J.; Rexiati, M.; Wang, W.-G.; Azhati, B.; Nuerrula, Y.; Wang, Y.-J. Functional Analysis of Serum MicroRNAs MiR-21 and MiR-106a in Renal Cell Carcinoma. Cancer Biomark. 2017, 18, 79–85. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, L.; Li, Y.; Huang, C.; Zeng, L.; Yang, J. Differential MicroRNA Expression in Renal Cell Carcinoma. Oncol. Lett. 2013, 6, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Lu, Y.; Xiao, Y.; Lu, Y. Upregulation of MiR-21 Expression Is a Valuable Predicator of Advanced Clinicopathological Features and Poor Prognosis in Patients with Renal Cell Carcinoma through the P53/P21-cyclin E2-Bax/Caspase-3 Signaling Pathway. Oncol. Rep. 2017, 37, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Deng, X.; Zhang, J. Identification of Dysregulated Serum MiR-508-3p and MiR-885-5p as Potential Diagnostic Biomarkers of Clear Cell Renal Carcinoma. Mol. Med. Rep. 2019, 20, 5075–5083. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Liu, K.; Pan, X.; Quan, J.; Zhou, L.; Li, Z.; Lin, C.; Xu, J.; Xu, W.; Guan, X.; et al. MiR-625-3p Promotes Migration and Invasion and Reduces Apoptosis of Clear Cell Renal Cell Carcinoma. Am. J. Transl. Res. 2019, 11, 6475–6486. [Google Scholar] [PubMed]
- Dias, F.; Teixeira, A.L.; Ferreira, M.; Adem, B.; Bastos, N.; Vieira, J.; Fernandes, M.; Sequeira, M.I.; Maurício, J.; Lobo, F.; et al. Plasmatic MiR-210, MiR-221 and MiR-1233 Profile: Potential Liquid Biopsies Candidates for Renal Cell Carcinoma. Oncotarget 2017, 8, 103315–103326. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.L.; Ferreira, M.; Silva, J.; Gomes, M.; Dias, F.; Santos, J.I.; Maurício, J.; Lobo, F.; Medeiros, R. Higher Circulating Expression Levels of MiR-221 Associated with Poor Overall Survival in Renal Cell Carcinoma Patients. Tumor Biol. 2014, 35, 4057–4066. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Lai, W.-J.; Zhu, W.-A.; Wang, H. MicroRNA Derived from Circulating Exosomes as Noninvasive Biomarkers for Diagnosing Renal Cell Carcinoma. Onco Targets Ther. 2020, 13, 10765–10774. [Google Scholar] [CrossRef] [PubMed]
- Wulfken, L.M.; Moritz, R.; Ohlmann, C.; Holdenrieder, S.; Jung, V.; Becker, F.; Herrmann, E.; Walgenbach-Brünagel, G.; von Ruecker, A.; Müller, S.C.; et al. MicroRNAs in Renal Cell Carcinoma: Diagnostic Implications of Serum MiR-1233 Levels. PLoS ONE 2011, 6, e25787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Ni, M.; Su, Y.; Wang, H.; Zhu, S.; Zhao, A.; Li, G. MicroRNAs in Serum Exosomes as Potential Biomarkers in Clear-Cell Renal Cell Carcinoma. Eur. Urol. Focus 2018, 4, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Li, X.; Chen, Z.; Wang, J.; Zhang, C.; Chen, X.; Peng, X.; Liu, K.; Zhao, L.; Lai, Y.; et al. A Three-MicroRNA Panel in Serum: Serving as a Potential Diagnostic Biomarker for Renal Cell Carcinoma. Pathol. Oncol. Res. 2020, 26, 2425–2434. [Google Scholar] [CrossRef]
- Redova, M.; Poprach, A.; Nekvindova, J.; Iliev, R.; Radova, L.; Lakomy, R.; Svoboda, M.; Vyzula, R.; Slaby, O. Circulating MiR-378 and MiR-451 in Serum Are Potential Biomarkers for Renal Cell Carcinoma. J. Transl. Med. 2012, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Hu, J.; Lu, M.; Gu, H.; Zhou, X.; Chen, X.; Zen, K.; Zhang, C.-Y.; Zhang, T.; Ge, J.; et al. A Panel of Five Serum MiRNAs as a Potential Diagnostic Tool for Early-Stage Renal Cell Carcinoma. Sci. Rep. 2015, 5, 7610. [Google Scholar] [CrossRef] [Green Version]
- Fedorko, M.; Stanik, M.; Iliev, R.; Redova-Lojova, M.; Machackova, T.; Svoboda, M.; Pacik, D.; Dolezel, J.; Slaby, O. Combination of MiR-378 and MiR-210 Serum Levels Enables Sensitive Detection of Renal Cell Carcinoma. Int. J. Mol. Sci. 2015, 16, 23382–23389. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Li, G.; Péoc’h, M.; Genin, C.; Gigante, M. Serum MiR-210 as a Novel Biomarker for Molecular Diagnosis of Clear Cell Renal Cell Carcinoma. Exp. Mol. Pathol. 2013, 94, 115–120. [Google Scholar] [CrossRef]
- Iwamoto, H.; Kanda, Y.; Sejima, T.; Osaki, M.; Okada, F.; Takenaka, A. Serum MiR-210 as a Potential Biomarker of Early Clear Cell Renal Cell Carcinoma. Int. J. Oncol. 2014, 44, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Wang, C.; Chen, K.; Wang, T.; Xing, J.; Zhang, X.; Wang, X. MiR-765 Functions as a Tumour Suppressor and Eliminates Lipids in Clear Cell Renal Cell Carcinoma by Downregulating PLP2. eBioMedicine 2020, 51, 102622. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Li, H.; Wang, J.; Peng, X.; Liu, K.; Zhao, L.; Zhang, C.; Chen, X.; Lai, Y.; Ni, L. Combination of Tumor Suppressor MiR-20b-5p, MiR-30a-5p, and MiR-196a-5p as a Serum Diagnostic Panel for Renal Cell Carcinoma. Pathol. Res. Pract. 2020, 216, 153152. [Google Scholar] [CrossRef]
- Lou, N.; Ruan, A.-M.; Qiu, B.; Bao, L.; Xu, Y.-C.; Zhao, Y.; Sun, R.-L.; Zhang, S.-T.; Xu, G.-H.; Ruan, H.-L.; et al. MiR-144-3p as a Novel Plasma Diagnostic Biomarker for Clear Cell Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 36.e7–36.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lei, T.; Xu, C.; Li, H.; Ma, W.; Yang, Y.; Fan, S.; Liu, Y. MicroRNA-187, down-Regulated in Clear Cell Renal Cell Carcinoma and Associated with Lower Survival, Inhibits Cell Growth and Migration Though Targeting B7-H3. Biochem. Biophys. Res. Commun. 2013, 438, 439–444. [Google Scholar] [CrossRef]
- Zhang, Q.; Di, W.; Dong, Y.; Lu, G.; Yu, J.; Li, J.; Li, P. High Serum MiR-183 Level Is Associated with Poor Responsiveness of Renal Cancer to Natural Killer Cells. Tumor Biol. 2015, 36, 9245–9249. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.G.; Tolkach, Y.; Deng, M.; Schmidt, D.; Perner, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Serum MiR-122-5p and MiR-206 Expression: Non-Invasive Prognostic Biomarkers for Renal Cell Carcinoma. Clin. Epigenetics 2018, 10, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanudet, E.; Wozniak, M.B.; Bouaoun, L.; Byrnes, G.; Mukeriya, A.; Zaridze, D.; Brennan, P.; Muller, D.C.; Scelo, G. Large-Scale Genome-Wide Screening of Circulating MicroRNAs in Clear Cell Renal Cell Carcinoma Reveals Specific Signatures in Late-Stage Disease. Int. J. Cancer 2017, 141, 1730–1740. [Google Scholar] [CrossRef]
- Du, M.; Giridhar, K.V.; Tian, Y.; Tschannen, M.R.; Zhu, J.; Huang, C.-C.; Kilari, D.; Kohli, M.; Wang, L. Plasma Exosomal MiRNAs-Based Prognosis in Metastatic Kidney Cancer. Oncotarget 2017, 8, 63703–63714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, F.; Teixeira, A.L.; Nogueira, I.; Morais, M.; Maia, J.; Bodo, C.; Ferreira, M.; Silva, A.; Vilhena, M.; Lobo, J.; et al. Extracellular Vesicles Enriched in Hsa-MiR-301a-3p and Hsa-MiR-1293 Dynamics in Clear Cell Renal Cell Carcinoma Patients: Potential Biomarkers of Metastatic Disease. Cancers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-G.; Zhu, Y.-W.; Wang, T.; Chen, B.; Xing, J.-C.; Xiao, W. MiR-483-5p Downregulation Contributed to Cell Proliferation, Metastasis, and Inflammation of Clear Cell Renal Cell Carcinoma. Kaohsiung J. Med. Sci. 2021, 37, 192–199. [Google Scholar] [CrossRef]
- Li, M.; Sha, Y.; Zhang, X. MiR-22 Functions as a Biomarker and Regulates Cell Proliferation, Cycle, Apoptosis, Migration and Invasion in Renal Cell Carcinoma. Int. J. Clin. Exp. Pathol. 2017, 10, 11425–11437. [Google Scholar] [PubMed]
- Sun, I.O.; Bae, Y.-U.; Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Choi, J.-S.; Doh, K.-O.; Kwon, S.H. Circulating MiRNAs in Extracellular Vesicles Related to Treatment Response in Patients with Idiopathic Membranous Nephropathy. J. Transl. Med. 2022, 20, 224. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of Exosomal MiRNA on Cancer Biology and Clinical Applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrolia, I.; Catani, V.; Oltrecolli, M.; Pipitone, S.; Vitale, M.G.; Masciale, V.; Chiavelli, C.; Bortolotti, C.A.; Nasso, C.; Grisendi, G.; et al. Chasing the Role of miRNAs in RCC: From Free-Circulating to Extracellular-Vesicle-Derived Biomarkers. Biology 2023, 12, 877. https://doi.org/10.3390/biology12060877
Mastrolia I, Catani V, Oltrecolli M, Pipitone S, Vitale MG, Masciale V, Chiavelli C, Bortolotti CA, Nasso C, Grisendi G, et al. Chasing the Role of miRNAs in RCC: From Free-Circulating to Extracellular-Vesicle-Derived Biomarkers. Biology. 2023; 12(6):877. https://doi.org/10.3390/biology12060877
Chicago/Turabian StyleMastrolia, Ilenia, Virginia Catani, Marco Oltrecolli, Stefania Pipitone, Maria Giuseppa Vitale, Valentina Masciale, Chiara Chiavelli, Carlo Augusto Bortolotti, Cecilia Nasso, Giulia Grisendi, and et al. 2023. "Chasing the Role of miRNAs in RCC: From Free-Circulating to Extracellular-Vesicle-Derived Biomarkers" Biology 12, no. 6: 877. https://doi.org/10.3390/biology12060877
APA StyleMastrolia, I., Catani, V., Oltrecolli, M., Pipitone, S., Vitale, M. G., Masciale, V., Chiavelli, C., Bortolotti, C. A., Nasso, C., Grisendi, G., Sabbatini, R., & Dominici, M. (2023). Chasing the Role of miRNAs in RCC: From Free-Circulating to Extracellular-Vesicle-Derived Biomarkers. Biology, 12(6), 877. https://doi.org/10.3390/biology12060877