Ganoderma Lucidum from Red Mushroom Attenuates Formaldehyde-Induced Liver Damage in Experimental Male Rat Model
Abstract
:Simple Summary
Abstract
1. Introduction
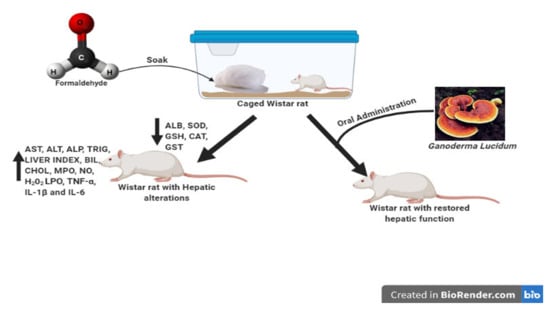
2. Materials and Methods
2.1. Fungi Material and Extraction
2.2. Chemicals
2.3. Animals Care
2.4. Experimental Design
2.5. Determination of Liver Function Parameters
2.6. Estimation of Antioxidant and Oxidative Stress Markers
2.7. Assessment of Inflammatory Biomarkers
2.8. Determination of Proinflammatory Cytokines
2.9. Histological Examination
2.10. Ethical Approval
2.11. Statistical Analyses
3. Results
3.1. G. lucidum Suppressed FA-Induced Reduction in Body and Organ Weight
3.2. G. lucidum Inhibits FA-Induced Alteration in Hepatic Function Enzymes
3.3. G. lucidum Attenuate FA-Induced Oxidative Damage in Rat Liver
3.4. G. lucidum Ameliorate FA-Induced Inflammation in Rat Liver
3.5. Histopathological Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Formalin Exposure: A Review of Known Health Hazards and the Role of Innovation in Improving Safety; Merit Medical Systems, Inc.: South Jordan, UT, USA, 2019.
- Repetto, R.; Baliga, S.S. Pesticides and the immune system: The public health risks. Executive summary. Cent. Eur. J. Public Health 1996, 4, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Ellenhorn, M.; Schonwald, G.; Ordog, J. Diagnosis and Treatment of Human Poisoning; Williams and Wikins: Los Angeles, CA, USA, 1997. [Google Scholar]
- ATSDR. Toxicological Profile for Formaldehyde. In ATSDR’s Toxicological Profiles; Agency for Toxic Substances and Diseases: Atlanta, GA, USA, 2002. [Google Scholar]
- WHO. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93.
- Cheng, G.; Shi, Y.; Sturla, S.J.; Jalas, J.R.; McIntee, E.J.; Villalta, P.W.; Wang, M.; Hecht, S.S. Reactions of formaldehyde plus acetaldehyde with deoxyguanosine and DNA: Formation of cyclic deoxyguanosine adducts and formaldehyde cross-links. Chem. Res. Toxicol. 2003, 16, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Restani, P.; Restelli, A.R.; Galli, C.L. Formaldehyde and hexamethylenetetramine as food additives: Chemical interactions and toxicology. Food Addit. Contam. 1992, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Beard, K.; Pourahmad, J.; Moridani, M.; Easson, E.; Poon, R.; Brien, P.J.O. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem. Biol. Interact. 2001, 132, 285–296. [Google Scholar] [CrossRef]
- Rumchev, K.B.; Spickett, J.T.; Bulsara, M.K.; Phillips, M.R.; Stick, S.M. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 2002, 20, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Nouh, W.G.; Selim, A.G. Toxopathological Studies on the Effect of Formalin and Copper Sulphate in Tilapia as A Commonly Used Disinfectant in Aquaculture. J. Appl. Environ. Biol. Sci. 2013, 3, 7–20. [Google Scholar]
- Takahashi, M.; Hasegawa, R.; Furukawa, F.; Toyoda, K.; Sato, H.; Hayashi, Y. Effects of ethanol, potassium metabisulfite, formaldehyde and hydrogen peroxide on gastric carcinogenesis in rats after initiation with n-methyl-n′-nitro-n-nitrosoguanidine. Jpn. J. Cancer Res. GANN 1986, 77, 118–124. [Google Scholar] [CrossRef]
- Franklin, P.; Dingle, P.; Stick, S. Raised exhaled nitric oxide in healthy children is associated with domestic formaldehyde levels. Am. J. Respir. Crit. Care Med. 2000, 161, 1757–1759. [Google Scholar] [CrossRef]
- Gowri Shankar, N.L.; Manavalan, R.; Venkappayya, D.; David Raj, C. Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl4-induced oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3182–3185. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Joshi, U.M.; Young, R.A.; Meydrech, E.F.; Mehendale, H.M. Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol. Pathol. 1989, 17, 494–505. [Google Scholar] [CrossRef] [Green Version]
- Ling-Sing Seow, S.; Naidu, M.; David, P.; Wong, K.H.; Sabaratnam, V. Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2013, 13, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Baptista, P.; Estevinho, L.M.; Ferreira, I.C.F.R. Effect of fruiting body maturity stage on chemical composition and antimicrobial activity of Lactarius sp. mushrooms. J. Agric. Food Chem. 2007, 55, 8766–8771. [Google Scholar] [CrossRef] [PubMed]
- Thu, Z.M.; Ko Myo, K.; Aung, H.T.; Clericuzio, M.; Armijos, C.; Vidari, G. Bioactive phytochemical constituents of wild edible mushrooms from Southeast Asia. Molecules 2020, 25, 1972. [Google Scholar] [CrossRef] [PubMed]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Keen, C.L.; Gershwini, M.E. Mushrooms, Tumors, and Immunity: An Update. Exp. Biol. Med. 2004, 229, 393–406. [Google Scholar] [CrossRef]
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J.A.; García-Lafuente, A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012, 130, 350–355. [Google Scholar] [CrossRef]
- Mori, K.; Kobayashi, C.; Tomita, T.; Inatomi, S.; Ikeda, M. Antiatherosclerotic effect of the edible mushrooms Pleurotus eryngii (Eringi), Grifola frondosa (Maitake), and Hypsizygus marmoreus (Bunashimeji) in apolipoprotein E-deficient mice. Nutr. Res. 2008, 28, 335–342. [Google Scholar] [CrossRef]
- Hu, S.H.; Wang, J.C.; Lien, J.L.; Liaw, E.T.; Lee, M.Y. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Appl. Microbiol. Biotechnol. 2006, 70, 107–113. [Google Scholar] [CrossRef]
- Sliva, D. Ganoderma lucidum (Reishi) in Cancer Treatment. Integr. Cancer Ther. 2003, 2, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Kim, H.W.; Lee, Y.S.; Shim, M.J.; Choi, E.C.; Kim, B. kak Studies on Safety of Ganoderma lucidum. Korean J. Mycol. 1986, 14, 49–59. [Google Scholar]
- Paterson, R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njoya, H.K.; Ofusori, D.A.; Nwangwu, S.C.; Amegor, O.F.; Akinyeye, A.J.; Abayomi, T.A. Histopathological effect of exposure of formaldehyde vapour on the trachea and lung of adult wistar rats. Int. J. Integr. Biol. 2009, 7, 160–165. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Hand Book of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glatathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Jollow, D.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Wolff, S.P. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994. [Google Scholar] [CrossRef]
- Granell, S.; Gironella, M.; Bulbena, O.; Panés, J.; Mauri, M.; Sabater, L.; Aparisi, L.; Gelpi, E.; Closa, D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 2003, 31, 525–530. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Oyebode, O.; Kandala, N.B.; Chilton, P.J.; Lilford, R.J. Use of traditional medicine in middle-income countries: A WHO-SAGE study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Schiff, N.D.; Giacino, J.T.; Kalmar, K.; Victor, J.D.; Baker, K.; Gerber, M.; Fritz, B.; Eisenberg, B.; O’Connor, J.; Kobylarz, E.J.; et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature 2007, 448, 600–603. [Google Scholar] [CrossRef]
- Lakshmi, B.; Ajith, T.A.; Jose, N.; Janardhanan, K.K. Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. J. Ethnopharmacol. 2006, 107, 297–303. [Google Scholar] [CrossRef]
- Sabbioni, G.; Turesky, R.J. Biomonitoring human albumin adducts: The past, the present, and the future. Chem. Res. Toxicol. 2017, 30, 332–366. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. Oxidative stress and liver cancer: Etiology and therapeutic targets. Oxid. Med. Cell. Longev. 2016, 2016, 7891574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beall, C.M.; Reichsman, A.B. Hemoglobin levels in a Himalayan high altitude population. Am. J. Phys. Anthropol. 1984, 63, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Payani, S.; Mamatha, C.; Chandraprakash, C.; Bhaskar, M. Protective role of (Bronco-T) against formaldehyde induced antioxidant, oxidative and histopathological changes in lung of male Wistar rats. Toxicol. Rep. 2019, 6, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jiang, X.; Deng, W.; Lai, Y.; Wu, M.; Zhang, Z. Antioxidant activities of Ganoderma lucidum polysaccharides and their role on DNA damage in mice induced by cobalt-60 gamma-irradiation. Food Chem. Toxicol. 2012, 50, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. Int. J. Mol. Med. 2011, 28, 1065–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohretoglu, D.; Huang, S. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anticancer Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, S.; Sabulal, B.; George, V.; Antony, K.R.; Janardhanan, K.K. Antitumor and anti-inflammatory activities of polysaccharides isolated from Ganoderma lucidum. Acta Pharm. 2011, 61, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagins, A.R.; Opriessnig, T.; Guenette, D.K.; Halbur, P.G.; Meng, X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008, 123, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.-S.; Beshbishy, A.M.; Wasef, L.; Elewa, Y.H.A.; El-Hack, M.E.A.; Taha, A.E.; Al-Sagheer, A.A.; Devkota, H.P.; Tufarelli, V. Uncaria tomentosa (Willd. ex Schult.) DC.: A Review on Chemical Constituents and Biological Activities. Appl. Sci. 2020, 10, 2668. [Google Scholar] [CrossRef] [Green Version]
- Ikram, M.; Beshbishy, A.M.; Kifayatullah, M.; Olukanni, A.; Zahoor, M.; Naeem, M.; Amin, M.; Shah, M.; Abdelaziz, A.S.; Ullah, R.; et al. Chemotherapeutic Potential of Carthamus oxycantha Root Extract as Antidiarrheal and In Vitro Antibacterial Activities. Antibiotics 2020, 9, 226. [Google Scholar] [CrossRef]
- El-Rahman, G.I.A.; Behairy, A.; Elseddawy, N.M.; Batiha, G.-S.; Hozzein, W.N.; Khodeer, D.M.; Abd-Elhakim, Y.M. Saussurea lappa Ethanolic Extract Attenuates Triamcinolone Acetonide-Induced Pulmonary and Splenic Tissue Damage in Rats via Modulation of Oxidative Stress, Inflammation, and Apoptosis. Antioxidants 2020, 9, 396. [Google Scholar] [CrossRef]
- Batiha, G.-S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]



| Control (g) | Formaldehyde (g) | Ganoderma lucidum | Ganoderma lucidum + Formaldehyde (g) | |
|---|---|---|---|---|
| Average Body weight | 42.35 ± 4.52 | 18.75 ± 5.45 a | 36.74 ± 6.32 | 25.45 ± 4.46 |
| Relative organ weight | 7.32 ± 0.47 | 4.76 ± 0.78 a | 8.76 ± 0.95 | 6.75 ± 0.52 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oluwafemi Adetuyi, B.; Olamide Okeowo, T.; Adefunke Adetuyi, O.; Abraham Adebisi, O.; Ogunlana, O.O.; Janet Oretade, O.; Marraiki, N.; Beshbishy, A.M.; N. Welson, N.; Batiha, G.E.-S. Ganoderma Lucidum from Red Mushroom Attenuates Formaldehyde-Induced Liver Damage in Experimental Male Rat Model. Biology 2020, 9, 313. https://doi.org/10.3390/biology9100313
Oluwafemi Adetuyi B, Olamide Okeowo T, Adefunke Adetuyi O, Abraham Adebisi O, Ogunlana OO, Janet Oretade O, Marraiki N, Beshbishy AM, N. Welson N, Batiha GE-S. Ganoderma Lucidum from Red Mushroom Attenuates Formaldehyde-Induced Liver Damage in Experimental Male Rat Model. Biology. 2020; 9(10):313. https://doi.org/10.3390/biology9100313
Chicago/Turabian StyleOluwafemi Adetuyi, Babatunde, Tolulope Olamide Okeowo, Oluwatosin Adefunke Adetuyi, Oluwaseun Abraham Adebisi, Olubanke Olujoke Ogunlana, Oyeyemi Janet Oretade, Najat Marraiki, Amany Magdy Beshbishy, Nermeen N. Welson, and Gaber El-Saber Batiha. 2020. "Ganoderma Lucidum from Red Mushroom Attenuates Formaldehyde-Induced Liver Damage in Experimental Male Rat Model" Biology 9, no. 10: 313. https://doi.org/10.3390/biology9100313
APA StyleOluwafemi Adetuyi, B., Olamide Okeowo, T., Adefunke Adetuyi, O., Abraham Adebisi, O., Ogunlana, O. O., Janet Oretade, O., Marraiki, N., Beshbishy, A. M., N. Welson, N., & Batiha, G. E. -S. (2020). Ganoderma Lucidum from Red Mushroom Attenuates Formaldehyde-Induced Liver Damage in Experimental Male Rat Model. Biology, 9(10), 313. https://doi.org/10.3390/biology9100313