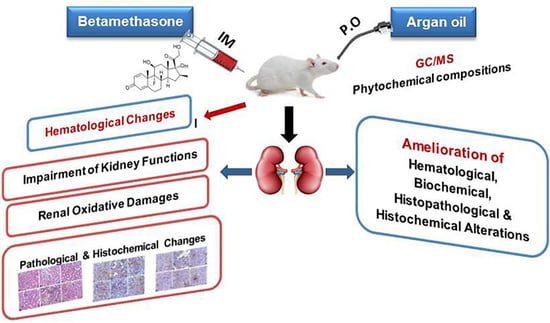
The Antioxidant, Anti-Apoptotic, and Proliferative Potency of Argan Oil against Betamethasone-Induced Oxidative Renal Damage in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of Argan Oil
2.3. Animals and Experimental Protocol
2.4. Sample Collection and Preparation
2.5. Investigation of Hematological Parameters
2.6. Assessment of Serum Renal Function Biomarkers
2.7. Assessment of Renal Oxidant/Antioxidant Biomarkers
2.8. Histopathological Examination
2.9. Immunohistochemical Investigation
2.10. Statistical Analysis
3. Results
3.1. The Phytochemical Components of Argan Oil
3.2. Argan Oil Ameliorated Betamethasone-Induced Changes in Erythrogram Parameters of Rats
3.3. Argan Oil Alleviated Betamethasone-Induced Changes in Leukogram and Blood Platelets of Rats
3.4. Argan Oil Reduced Betamethasone-Induced Elevation in Serum Kidney Functions Biomarkers of Rats
3.5. Argan Oil Improved Betamethasone-Induced Alterations in Renal Oxidant/Antioxidant Biomarkers of Rats
3.6. Argan Oil Ameliorated Betamethasone-Induced Alterations in Renal Histoarchitecture of Rats
3.7. Argan Oil Modulated Betamethasone-Induced Increase of Bax and Caspase-3 and Decrease of Bcl-2 and PCNA Expressions in Renal Tissues of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fietta, P.; Fietta, P.; Delsante, G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin. Neurosci. 2009, 63, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Akalestou, E.; Genser, L.; Rutter, G.A. Glucocorticoid Metabolism in Obesity and Following Weight Loss. Front. Endocrinol. 2020, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirwan, J.R.; Clarke, L.; Hunt, L.P.; Perry, M.G.; Straub, R.H.; Jessop, D.S. Effect of novel therapeutic glucocorticoids on circadian rhythms of hormones and cytokines in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2010, 1193, 127–133. [Google Scholar] [CrossRef]
- Edwards, C. Sixty Years after Hench-Corticosteroids and Chronic Inflammatory Disease. J. Clin. Endocrinol. 2012, 97, 1443–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorthy, S.; Disease, J.C.-R. Corticosteroids: Mechanisms of action in health and disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäcke, H.; Döcke, W.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Fardet, L.; Petersen, I.; Nazareth, I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am. J. Psychiatry 2012, 169, 491–497. [Google Scholar] [CrossRef]
- Amar, M.; Shama, I.A.; Enaia, A.A.; Hind, A.E.O.; Hager, A.M. Effects of various levels of oral doses dexamethasone (Al-nagma) abused as cosmetic by Sudanese women on Wistar rats. J. Med. Sci. 2013, 13, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Fardet, L.; Fève, B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs 2014, 74, 1731–1745. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Zheng, L.; Liu, Y.; Liu, G.; Li, N.; Wang, K.; Zou, L.; Wu, T.; Qin, L.; et al. Glucocorticoid-Induced Osteoporosis in Growing Rats. Calcif. Tissue Int. 2014, 95, 362–373. [Google Scholar] [CrossRef]
- Eken, H.; Ozturk, H.; Ozturk, H.; Buyukbayram, H. Dose-related effects of dexamethasone on liver damage due to bile duct ligation in rats. World J. Gastroenterol. 2006, 12, 5379–5383. [Google Scholar] [CrossRef] [PubMed]
- Polycarp, T.N.; Yusoff, S.M.; Benjamin, E.O.; Salisi, S.M.; Bejo, S.-K. Influence of dexamethasone-induced stress on oxidative stress biomarkers in non-pregnant does experimentally infected with Brucellamelitensis. Comp. Clin. Pathol. 2017, 26, 423–435. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Ichiseki, T.; Ueda, S.; Ueda, Y.; Shimazaki, M.; Kaneuji, A.; Kawahara, N. Mitochondrial stress and redox failure in steroid-associated osteonecrosis. Int. J. Med. Sci. 2018, 15, 205. [Google Scholar] [CrossRef] [Green Version]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [Green Version]
- El Abbassi, A.; Khalid, N.; Zbakh, H.; Ahmad, A. Physicochemical Characteristics, Nutritional Properties, and Health Benefits of Argan Oil: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1401–1414. [Google Scholar] [CrossRef]
- Monfalouti, H.E.; Guillaume, D.; Denhez, C.; Charrouf, Z. Therapeutic potential of argan oil: A review. J. Pharm. Pharmacol. 2010, 62, 1669–1675. [Google Scholar] [CrossRef]
- López, L.C.; Cabrera-Vique, C.; Venegas, C.; García-Corzo, L.; Luna-Sánchez, M.; Acuña-Castroviejo, D.; Escames, G. Argan oil-contained antioxidants for human mitochondria. Nat. Prod. Commun. 2013, 8, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Charrouf, Z.; Guillaume, D. Argan oil and other argan products; use in dermocosmetology. Eur. J. Lipid Sci. Technol. 2011, 113, 403–408. [Google Scholar] [CrossRef]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; Cherkaoui-Malki, M. Protective effect of argan and olive oils against LPS-induced oxidative stress and inflammation in mice livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şekeroğlu, Z.; Aydın, B.; Şekeroğlu, V. Argan oil reduces oxidative stress, genetic damage and emperipolesis in rats treated with acrylamide. Biomed. Pharmacother. 2017, 94, 873–920. [Google Scholar] [CrossRef]
- Aydın, B. Effects of Argan oil on the mitochondrial function, antioxidant system and the activity of NADPH- generating enzymes in acrylamide treated rat brain. Biomed. Pharmacother. 2017, 87, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Saber, T.M.; Mansour, M.F.; Abdelaziz, A.S.; Mohamed, R.M.; Fouad, R.A.; Arisha, A.H. Argan oil ameliorates sodium fluoride-induced renal damage via inhibiting oxidative damage, inflammation, and intermediate filament protein expression in male rats. Environ. Sci. Pollut. Res. 2020, 27, 30426–30436. [Google Scholar] [CrossRef]
- Tsuji, F.; Sawa, K.; Ikuse, T.; Shirasawa, E. The effects of betamethasone derivatives on endotoxin-induced uveitis in guinea pigs. Inflamm. Res. 1997, 46, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Grindem, C.B. Schalm’s Veterinary Hematology, Editors: Douglas, J.; Weiss, K. Jane Wardrop. Vet. Clin. Pathol. 2011, 2, 270. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Elsevier: Oxford, UK, 2019; pp. 173–186. [Google Scholar]
- Oyouni, A.A.; Saggu, S.H.; Tousson, E.; Rehman, H. Immunosuppressant drug tacrolimus induced mitochondrial nephrotoxicity, modified PCNA and Bcl-2 expression attenuated by Ocimumbasilicum L. in CD1 mice. Toxicol. Rep. 2018, 5, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Shalkami, A.S.; Khalaf, M.M.; Mohamed, W.R. The impact of Keap1/Nrf2, P38MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yu, T.; Qi, X.; Gao, J.; Huang, K.; He, X.; Luo, H.; Xu, W.; Manderville, R.A.; Pfohl-Leszkowicz, A. Limited Link between Oxidative Stress and Ochratoxin A-Induced Renal Injury in an Acute Toxicity Rat Model. Toxins 2016, 8, 373. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Dhar, D.K.; Yamaguchi, E.; Maruyama, S.; Sato, T.; Hayashi, H.; Ono, T.; Yamanoi, A.; Kohno, H.; Nagasue, N. Coexpression of Inducible Nitric Oxide Synthase and COX-2 in Hepatocellular Carcinoma and Surrounding Liver: Possible Involvement of COX-2 in the Angiogenesis of Hepatitis C Virus-positive Cases. Clin. Can. Res. 2001, 7, 1325–1332. [Google Scholar]
- Locatelli, F.; Andrulli, S.; Memoli, B.; Maffei, C.; Del Vecchio, L.; Aterini, S.; Cianciaruso, B. Nutritional- inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol. Dial. Transplant. 2006, 21, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Krantz, S.; Jacobson, L. Morphological identity of the hematopoietic stem cell. In Erythropoietin and the Regulation of Erythropoiesis; University of Chicago Press: Chicago, IL, USA, 1970; pp. 98–102. [Google Scholar] [CrossRef]
- Kuter, D.J. Biology and chemistry of thrombopoietic agents. Semin. Hematol. 2010, 47, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti-and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation 2015, 22, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Rasania, S.; Dadhaniya, P.; Patel, C.; Vachhani, K. Assesment of 28 days repeated adminstration toxicity profile of dexamethasone palmitate injection. J. Nepal Pharm. Assoc. 2014, 27, 9–19. [Google Scholar] [CrossRef] [Green Version]
- O’Shaunessy, K. Principles of Clinical Pharmacology and Drug Therapy. Oxf. Textb. Med. 2010, 1, 1450–1476. [Google Scholar]
- Singh, N.P.; Ganguli, A.; Prakash, A. Drug-induced kidney diseases. J. Assoc. Physicians India 2003, 51, 970–979. [Google Scholar]
- Jain, J.; Legan, S.K.; Alhamoud, I.; Gattineni, J.; Baum, M. Effect of sex on glomerular filtration rate in programmed rats by prenatal dexamethasone. Physiol. Rep. 2019, 7, e14154. [Google Scholar] [CrossRef] [Green Version]
- Adedara, I.A.; Teberen, R.; Ebokaiwe, A.P.; Ehwerhemuepha, T.; Farombi, E.O. Induction of Oxidative Stress in Liver and Kidney of Rats Exposed to Nigerian Bonny Light Crude Oil. Environ. Toxicol. 2012, 27, 372–379. [Google Scholar] [CrossRef]
- Verhaeghe, J.; van Bree, R.; Van Herck, E. Oxidative stress after antenatal betamethasone: Acute downregulation of glutathione peroxidase-3. Early Hum. Dev. 2009, 85, 767–771. [Google Scholar] [CrossRef]
- Sato, H.; Takahashi, T.; Sumitani, K.; Takatsu, H.; Urano, S. Glucocorticoid Generates ROS to Induce Oxidative Injury in the Hippocampus, Leading to Impairment of Cognitive Function of Rats. J. Clin. Biochem. Nutr. 2010, 47, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Pedrana, G.; Viotti, M.H.; Souza, E.; Sloboda, D.; Martin, G.B.; Cavestany, D.; Ortega, H.H. Apoptosis-Related Protein Expression During Pre-and Post-Natal Testicular Development After Administration of Glucocorticoid in utero in the Sheep. Reprod. Domest. Anim. 2013, 48, 795–802. [Google Scholar] [CrossRef]
- Kuranaga, E. Beyond apoptosis: Caspase regulatory mechanisms and functions in vivo. Genes Cells 2012, 17, 83–97. [Google Scholar] [CrossRef]
- Singh, L.; Pushker, N.; Saini, N.; Sen, S.; Sharma, A.; Bakhshi, S.; Chawla, B.; Kashyap, S. Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins in human retinoblastoma. Clin. Exp. Ophthalmol. 2015, 43, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Bcl-2 family: Life-or-death switch. FEBS Lett. 2000, 466, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.Q.; Jia, Y.; Chen, G. Possible involvement of cathepsin B/D and caspase-3 in deferoxamine-related neuroprotection of early brain injury after subarachnoid haemorrhage in rats. Neuropathol. Appl. Neurobiol. 2014, 40, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000, 102, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Yiran, Z.; Chenyang, J.; Jiajing, W.; Yan, Y.; Jianhong, G.; Jianchun, B.; Zongping, L. Oxidative stress and mitogen-activated protein kinase pathways involved in cadmium-induced BRL 3A cell apoptosis. Oxidative Med. Cell. Longev. 2013, 2013, 516051. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ueta, E.; Kimura, T.; Yamamoto, T.; Osaki, T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.S. Metals and apoptosis: Recent developments. J. Trace Elem. Med. Biol. 2008, 22, 262–284. [Google Scholar] [CrossRef]
- Chuang, Y.; Chuang, W.; Huang, C. Myocyte apoptosis in the pathogenesis of ureteral damage in rats with obstructive uropathy. Urology 2001, 58, 463–470. [Google Scholar] [CrossRef]
- Mackay, D.; Cowan-Ellsberry, C.E.; Powell, D.E.; Woodburn, K.B.; Xu, S.; Kozerski, G.E.; Kim, J. Decamethylcyclopentasiloxane (D5) environmental sources, fate, transport, and routes of exposure. Environ. Toxicol. Chem. 2015, 34, 2689–2702. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Maina, R.; Tumiatti, V.; Sarzanini, C.; De Carlo, R.M. Simultaneous determination of passivator and antioxidant additives in insulating mineral oils by high-performance liquid chromatography. J. Liquid Chromatogr. Relat. Technol. 2015, 38, 15–19. [Google Scholar] [CrossRef]
- Kurtz, A.; Wenger, R.H.; Eckardt, K.U. Hematopoiesis and the Kidney. In Seldin and Giebisch’s the Kidney Physiology and Pathophysiology; Alpern Robert, J., Caplan Michael, J., Moe Orson, W., Eds.; Elsevier: New York, NY, USA, 2013; pp. 3087–3124. [Google Scholar]
- Bakour, M.; Soulo, N.; Hammas, N.; El Fatemi, H.; Aboulghazi, A.; Taroq, A.; Abdellaoui, A.; Al-Waili, N.; Lyoussi, B. The Antioxidant Content and Protective Effect of Argan Oil and Syzygium aromaticum Essential Oil in Hydrogen Peroxide-Induced Biochemical and Histological Changes. Int. J. Mol. Sci. 2018, 19, 610. [Google Scholar] [CrossRef] [Green Version]
- Alahmadi, A.A.; EL-Hag, G.A.; Rabah, S.O.; Al-Baqami, N.M.; Zeid, I.A.; Al-Attar, A.M. Physiological and Histopathological Effect of Argan (Arganiaspinosa L.) Seed Oil on Kidney Male Rats Exposed to Lead. World Appl. Sci. J. 2018, 36, 733–741. [Google Scholar] [CrossRef]
- Sour, S.; Belarbi, M.; Sari, N.; Benammar, C.H.; Baghdad, C.H.; Visioli, F. Argan oil reduces, in rats, the high fat diet-induced metabolic effects of obesity. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Liapis, H.; Hammerman, M.R. Expression of bcl-2 and bax in regenerating rat renal tubules following ischemic injury. Am. J. Physiol. Ren. Physiol. 1997, 272, F640–F647. [Google Scholar] [CrossRef] [PubMed]
- Gerster, H. Anticarcinogenic effect of common carotenoids. Int. J. Vitam. Nutr. Res. 1993, 63, 93. [Google Scholar]
- Jafari, A.; Pourrazi, H.; Nikookheslat, S.; Baradaran, B. Effect of Exercise Training on Bcl-2 and Bax Gene Expression in the Rat Heart. Gene Cell Tissue 2015, 2, e60174. [Google Scholar] [CrossRef] [Green Version]







| Compound Name | RT/min | Area % | MW | MF |
|---|---|---|---|---|
| Cyclopentasiloxane, decamethyl- | 5.05 | 28.3 | 370 | C10H30O5Si5 |
| Decyloxyamine | 6.9 | 0.49 | 168 | C10H23NO |
| Nonadecane | 7.07 | 0.45 | 268 | C19H40 |
| Hexadecane | 8.62 | 0.92 | 226 | C16H34 |
| Phytol | 8.76 | 0.52 | 296 | C20H40O |
| 2-Piperidinone, N-[4-bromo-n-butyl]- | 8.85 | 0.5 | 233 | C9H16BrNO |
| Tridecane, 3-methyl- | 8.97 | 0.41 | 198 | C14H30 |
| 7-Hexadecenal, (Z)- | 9.67 | 0.79 | 238 | C16H30O |
| 8-Hexadecenal, 14-methyl-, (Z)- | 9.78 | 0.54 | 252 | C17H32O |
| Phenol, 2,6-BIS(1,1-Dimethylethyl)-4 –Methyl | 11.55 | 13.13 | 220 | C15H24O |
| Pentalene, Octahydro-1-(2-Octyldecyl) | 12.24 | 0.29 | 362 | C26H50 |
| 1,2-15,16-Diepoxyhexadecane | 12.33 | 0.72 | 254 | C16H30O2 |
| 2,5-Furandione, 3-(Dodecenyl) Dihydro | 15.05 | 0.35 | 266 | C16H26O3 |
| 14-á-H-Pregna | 15.73 | 0.6 | 288 | C21H36 |
| 12-Methyl-E,E-2,13-octadecadien-1- ol | 16.83 | 1.68 | 280 | C19H36O |
| Parameters | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | BM | AO/0.5 | AO/1 | BM+AO/0.5 | BM+AO/1 | |
| RBCs (×106) | 8.04 ± 0.12 a | 6.81 ± 0.59 b | 7.89 ± 0.20 a | 7.83 ± 0.31 a | 7.26 ± 0.11 ab | 7.54 ± 0.14 ab |
| Hb (g/dl) | 15.90 ± 0.05 a | 12.88 ± 1.16 b | 15.40 ± 0.54 a | 15.60 ± 0.21 a | 14.72 ± 0.058 a | 14.88 ± 0.097 a |
| PCV (%) | 46.00 ± 0.63 a | 41.00 ± 2.79 b | 45.80 ± 0.58 a | 46.00 ± 0.63 a | 44.40 ± 0.81 ab | 45.00 ± 0.71 a |
| MCV (fl) | 57.29 ± 1.46 a | 60.79 ± 1.99 a | 58.20 ± 1.64 a | 59.06 ± 1.84 a | 61.21 ± 1.60 a | 60.38 ± 1.66 a |
| MCH (pg) | 19.79 ± 0.32 a | 18.89 ± 0.23 a | 19.51 ± 0.39 a | 20.05 ± 0.84 a | 20.29 ± 0.34 a | 19.77 ± 0.43 a |
| MCHC (%) | 34.59 ± 0.49 a | 31.19 ± 0.99 b | 33.63 ± 1.17 ab | 33.94 ± 0.71 a | 33.20 ± 0.66 ab | 33.09 ± 0.52 ab |
| Parameters | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | BM | AO/0.5 | AO/1 | BM + AO/0.5 | BM + AO/1 | |
| TWBCs (×103) | 11.50 ± 0.04 b | 12.68 ± 0.08 a | 11.54 ± 0.27 b | 11.50 ± 0.24 b | 11.36 ± 0.12 b | 11.44 ± 0.11 b |
| Neutrophils (×103) | 3.13 ± 0.04 bc | 4.77 ± 0.11 a | 3.12 ± 0.21 bc | 2.99 ± 0.11 c | 3.48 ± 0.07 b | 3.36 ± 0.07 b |
| Lymphocytes (×103) | 7.82 ± 0.050 a | 6.97 ± 0.057 b | 7.77 ± 0.18 a | 7.87 ± 0.19 a | 7.18 ± 0.11 b | 7.35 ± 0.13 b |
| Monocytes (×103) | 0.53 ± 0.03 d | 0.94 ± 0.03 a | 0.62 ± 0.03 c | 0.62 ± 0.03 c | 0.70 ± 0.02 bc | 0.73 ± 0.03 b |
| Eosinophils (×103) | 0.02 ± 0.02 a | 0.00 ± 0.00 a | 0.02 ± 0.02 a | 0.02 ± 0.02 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Platelets (×103) | 353.0 ± 0.45 a | 335.6 ± 0.51c | 354.0 ± 1.05 a | 353.4 ± 0.81 a | 349.2 ± 0.97 b | 349.4 ± 1.47 b |
| Lesions | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | BM | AO/0.5 | AO/1 | BM+AO/0.5 | BM+AO/1 | |
| Vascular Changes | − | ++++ | − | − | + | + |
| Degenerative Changes | − | +++ | − | − | + | + |
| Inflammatory Changes | − | ++ | − | − | + | + |
| IHC | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | BM | AO/0.5 | AO/1 | BM + AO/0.5 | BM + AO/1 | |
| Bax (% of positive cells/HPF) | 9.85 ± 0.61 c | 28.24 ± 1.43 a | 8.37 ± 0.54 c | 7.25 ± 0.31 c | 15.94 ± 1.01 b | 14.21 ± 1.05 b |
| Bcl-2 (% of positive cells/HPF) | 27.33 ± 0.88 a | 5.36 ± 0.17 b | 24.35 ± 0.54 a | 25.40 ± 0.44 a | 28.67 ± 0.50 a | 28.82 ± 0.13 a |
| Bax/Bcl-2 Ratio (% of positive cells/HPF) | 0.36 ± 0.06 b | 5.27 ± 0.73 a | 0.34 ± 0.02 b | 0.29 ± 0.03 b | 0.56 ± 0.03 b | 0.49 ± 0.02 b |
| Caspase-3 (% of positive cells/HPF) | 7.07 ± 0.72 c | 39.87 ± 2.00 a | 6.42 ± 0.36 c | 5.49 ± 0.64 c | 18.48 ± 1.73 b | 15.75 ± 1.92 b |
| PCNA (% of positive cells/HPF) | 32.75 ± 0.39 a | 10.67 ± 0.36 b | 33.35 ± 0.49 a | 34.13 ± 0.46 a | 30.48 ± 0.43 a | 31.70 ± 0.31 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orabi, S.H.; Allam, T.S.; Shawky, S.M.; Tahoun, E.A.E.-a.; Khalifa, H.K.; Almeer, R.; Abdel-Daim, M.M.; El-Borai, N.B.; Mousa, A.A. The Antioxidant, Anti-Apoptotic, and Proliferative Potency of Argan Oil against Betamethasone-Induced Oxidative Renal Damage in Rats. Biology 2020, 9, 352. https://doi.org/10.3390/biology9110352
Orabi SH, Allam TS, Shawky SM, Tahoun EAE-a, Khalifa HK, Almeer R, Abdel-Daim MM, El-Borai NB, Mousa AA. The Antioxidant, Anti-Apoptotic, and Proliferative Potency of Argan Oil against Betamethasone-Induced Oxidative Renal Damage in Rats. Biology. 2020; 9(11):352. https://doi.org/10.3390/biology9110352
Chicago/Turabian StyleOrabi, Sahar Hassan, Tamer S. Allam, Sherif Mohamed Shawky, Enas Abd El-aziz Tahoun, Hanem K. Khalifa, Rafa Almeer, Mohamed M. Abdel-Daim, Nermeen Borai El-Borai, and Ahmed Abdelmoniem Mousa. 2020. "The Antioxidant, Anti-Apoptotic, and Proliferative Potency of Argan Oil against Betamethasone-Induced Oxidative Renal Damage in Rats" Biology 9, no. 11: 352. https://doi.org/10.3390/biology9110352
APA StyleOrabi, S. H., Allam, T. S., Shawky, S. M., Tahoun, E. A. E. -a., Khalifa, H. K., Almeer, R., Abdel-Daim, M. M., El-Borai, N. B., & Mousa, A. A. (2020). The Antioxidant, Anti-Apoptotic, and Proliferative Potency of Argan Oil against Betamethasone-Induced Oxidative Renal Damage in Rats. Biology, 9(11), 352. https://doi.org/10.3390/biology9110352