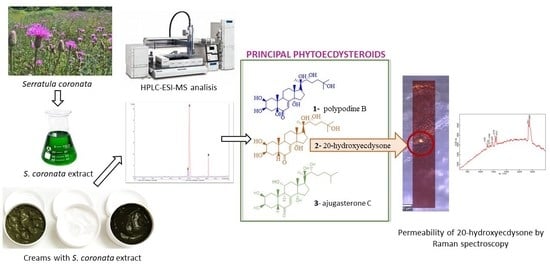
Analysis of Cosmetic Products Containing Serratula coronata Herb Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Analysis
2.1.1. Analysis of the Chemical Content of the Extract Using Thin-Layer Chromatography
2.1.2. Analysis of the Dominant Phytoecdysteroids in the Creams Using HPLC-ESI-MS
2.2. Physical and Chemical Analysis of the Creams
2.2.1. Chemical and Microbiological Stability of the Creams
Chemical Stability
Microbiological Purity
Viscosity and pH
2.3. Permeability of 20-Hydroxyecdysone by Raman Spectroscopy in an In Vitro Model
3. Materials and Methods
3.1. Standard and Chemicals
3.2. Material Extraction [35]
3.2.1. Analysis of the Chemical Composition of the Extract Using Thin-Layer Chromatography
3.2.2. Analysis of the Dominant Phytoecdysteroids in the S. Coronata Extract Using Liquid Chromatography–Electrospray Ionization–Mass Spectrometry (HPLC-ESI-MS)
3.3. Cream Preparation
3.4. Viscosity and pH
3.5. Chemical Stability
3.6. Microbiological Stability
3.7. Analysis of the Dominant Phytoecdysteroids Using HPLC-ESI-MS
3.7.1. Sample Preparation
3.7.2. Conditions of the Analysis
3.8. Permeability of 20-Hydroxyecdysone as Measured by Raman Spectroscopy in An In Vitro Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kroma, A.; Pawlaczyk, M.; Feliczak-Guzik, A.; Urbańska, M.; Jenerowicz, D.; Seraszek-Jaros, A.; Kikowska, M.; Gornowicz-Porowska, J. Phytoecdysteroids from Serratula coronata L. for Psoriatic Skincare. Molecules 2022, 27, 3471. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Singh, M.; Devi, G.; Chaturvedi, R. Herbal Medicine and Biotechnology for the Benefit of Human Health. In Animal Biotechnology: Models in Discovery and Translation; Verma, A.S., Sing, A., Eds.; Elsevier Science Publishing Co. Inc.: San Diego, CA, USA, 2014; pp. 563–575. [Google Scholar]
- Olennikov, D.N. Metabolites of Serratula L. and Klasea Cass. (Asteraceae): Diversity, Separation Methods, and Bioactivity. Separations 2022, 9, 448. [Google Scholar] [CrossRef]
- Ivashchenko, I.; Rakhmetov, D.B. Biomorphological Features of Serratula coronata L. (Asteraceae) Introduced in ZHNAEU’s Botanical Garden. Mod. Phytomorphol. 2016, 10, 69–80. [Google Scholar]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The Phytochemical, Biological, and Medicinal Attributes of Phytoecdysteroids: An Updated Review. Acta Pharm. Sin. B 2021, 11, 1740–1766. [Google Scholar] [CrossRef] [PubMed]
- Laekeman, G.; Vlietinck, A. Phytoecdysteroids: Phytochemistry and Pharmacological Activity. In Natural Products: Phyto-Chemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3827–3849. [Google Scholar]
- Ványolós, A.; Báthori, M. New Perspectives in the Analysis of Ecdysteroids: A Promising Group of Biologically Active Compounds. Curr. Pharm. Anal. 2008, 4, 162–175. [Google Scholar] [CrossRef]
- Garg, A.; Sharma, R.; Dey, P. Analysis of Triterpenes and Triterpenoids. In Recent Advances in Natural Products Analysis; Silva, A.S., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–426. [Google Scholar]
- Odinokov, V.N.; Galyautdinov, I.V.; Nedopekin, D.V.; Khalilov, L.M.; Shashkov, A.S.; Kachalab, V.V.; Dinanc, L.; Lafont, R. Phytoecdysteroids from the juice of Serratula coronata L. (Asteraceae). Insect Mol. Biol. 2002, 32, 161–165. [Google Scholar] [CrossRef]
- Ghosh, D.; Laddha, K.S. Extraction and Monitoring of Phytoecdysteroids Through HPLC. J. Chromatogr. Sci. 2006, 44, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Michalek, I.M.; John, S.M.; Caetano Dos Santos, F.L. Microbiological Contamination of Cosmetic Products—Observations from Europe, 2005–2018. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2151–2157. [Google Scholar] [CrossRef]
- Kim, H.W.; Seok, Y.S.; Cho, T.J.; Rhee, M.S. Risk Factors Influencing Contamination of Customized Cosmetics Made On-the-Spot: Evidence from the National Pilot Project for Public Health. Sci. Rep. 2020, 10, 1561. [Google Scholar] [CrossRef] [Green Version]
- Choubey, S.; Godbole, S. Methods for Evaluation of Microbiological Safety, Guidelines Governing the Quality and Survey on Microbial Contamination of Commercial Cosmetic Products—A Review. WJPMR 2017, 3, 85–94. [Google Scholar]
- Noor, R.; Zerin, N.; Das, K.K.; Nitu, L.N. Safe usage of cosmetics in Bangladesh: A quality perspective based on microbiological attributes. J. Biol. Res. Thessalon. 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, D.; Towle, K.; Fung, E.; Novick, R.; Paustenbach, D.; Monnot, A. Chemical Stability Analysis of Hair Cleansing Conditioners under High-Heat Conditions Experienced during Hair Styling Processes. Cosmetics 2018, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Jamrógiewicz, M.; Merchel, M. A History of the Physical and Chemical Stability of Pharmaceuticals—A Review. Acta Pol. Pharm. 2018, 75, 297–304. [Google Scholar]
- Wirotesangthong, M. Microbiological Limits in Cosmetics. Isan J. Pharm. Sci. 2021, 17, 1–22. [Google Scholar] [CrossRef]
- Registration of Medicinal Products, Medical Devices and Biocidal Products. Polish Pharmacopoeia, 12th ed.; Registration of Medicinal Products, Medical Devices and Biocidal Products: Warsaw, Poland, 2020; Volume I.
- Proksch, E. pH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Chan, A.; Mauro, T. Acidification in the Epidermis and the Role of Secretory Phospholipases. Dermato-Endocrinol. 2011, 3, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Nagoba, B.S.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic Environment and Wound Healing: A Review. Wounds 2015, 27, 5–11. [Google Scholar]
- Davies, A.; Amin, S. Rheology of Cosmetic Products: Surfactant Mesophases, Foams and Emulsions. J. Cosmet. Sci. 2020, 71, 481–496. [Google Scholar]
- Gräbner, D.; Hoffmann, H. Rheology of Cosmetic Formulations. In Cosmetic Science and Technology: Theoretical Principles and Applications; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 471–488. [Google Scholar]
- Moravkova, T.; Stern, P. Rheological and Textural Properties of Cosmetic Emulsions. Appl. Rheol. 2011, 21, 1–6. [Google Scholar] [CrossRef]
- Sarkar, D.K. Creams and Ointments. In Pharmaceutical Emulsions: A Drug Developer’s Toolbag; Sarkar, D.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 69–76. [Google Scholar]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Shabbir, M.; Ali, S.; Shahid, N.; Rehman, K.; Umair, A.; Moosa, R. Formulation Considerations And Factors Affecting Transdermal Drug Delivery System. Int. J. Pharm. Integr. Sci. 2014, 2, 20–35. [Google Scholar]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human Skin Penetration of Hyaluronic Acid of Different Molecular Weights as Probed by Raman Spectroscopy. Skin. Res. Technol. 2015, 22, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Buniam, J.; Chukijrungroat, N.; Rattanavichit, Y.; Surapongchai, J.; Weerachayaphorn, J.; Bupha-Intr, T.; Saengsirisuwan, V. 20-Hydroxyecdysone Ameliorates Metabolic and Cardiovascular Dysfunction in High-Fat-High-Fructose-Fed Ovariectomized Rats. BMC Complement. Med. Ther. 2022, 20, 140. [Google Scholar] [CrossRef]
- Festucci-Buselli, R.A.; Contim, L.A.S.; Barbosa, L.C.A.; Stuart, J.; Otoni, W.C. Biosynthesis and Potential Functions of the Ecdysteroid 20-Hydroxyecdysone—A Review. Canad. J. Bot. 2008, 86, 978–987. [Google Scholar] [CrossRef]
- Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Adamski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the S. Coronata Herb with Antiseborrheic Dermatitis Activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. [Google Scholar] [CrossRef]
- Nowak, G.; Nawrot, J.; Latowski, K. Arbutin in Serratula quinquefolia M.B. (Asteraceae). Acta Soc. Bot. Pol. 2009, 78, 137–140. [Google Scholar] [CrossRef]
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef] [Green Version]
- González-González, O.; Ramirez, I.O.; Ramirez, B.I.; O’Connell, P.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Drug Stability: ICH versus Accelerated Predictive Stability Studies. Pharmaceutics 2022, 14, 2324. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines EDQM. European Pharmacopoeia, 7th ed.; The European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2010; pp. 163–167, 519–520. [Google Scholar]
- ICH. Q2 (R2): Validation of Analytical Procedures–Text and Methodology. In Proceedings of the International Conference on Harmonization (ICH), Geneva, Switzerland, 31 March 2022. [Google Scholar]








| Creams | Total Aerobic Count Test Result | Total Yeast and Mold Count Test Result | Method |
|---|---|---|---|
| 0—Lekobaza® | <10 CFU/g | <10 CFU/g | According to the Polish Pharmacopoeia XII |
| 1—Lekobaza® + S. coronata extract | <10 CFU/g | <10 CFU/g | |
| 2—Lekobaza® + salicylic acid | <10 CFU/g | <10 CFU/g | |
| 3—Lekobaza® + salicylic acid + S. coronata extract | <10 CFU/g | <10 CFU/g |
| Creams | pH M ± SD | Viscosity (mPA × S) M ± SD |
|---|---|---|
| 0—Lekobaza | 5.03 ± 0.05 | 152,900 ± 33,517 |
| 1—Lekobaza® + S. coronata extract | 4.30 ± 0.06 | 121,737 ± 1744 |
| 2—Lekobaza® + salicylic acid | 3.07 ± 0.12 | 194,200 ± 17665 |
| 3—Lekobaza® + salicylic acid | 2.47 ± 0.06 | 134,607 ± 2119 |
| +S. coronata extract |
| Time (min) | Mobile Phase | |
|---|---|---|
| H2O + 0.1% FA (%) | ACN + 0.1% FA (%) | |
| 0 | 90.0 | 10.0 |
| 1 | 90.0 | 10.0 |
| 12 | 87.5 | 12.5 |
| 14 | 87.5 | 12.5 |
| 20 | 55.0 | 45.0 |
| 22 | 55.0 | 45.0 |
| 24 | 10.0 | 90.0 |
| 26 | 10.0 | 90.0 |
| 28 | 90.0 | 10.0 |
| 35 | 90.0 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kroma, A.; Feliczak-Guzik, A.; Pawlaczyk, M.; Osmałek, T.; Urbańska, M.; Micek, I.; Nawrot, J.; Gornowicz-Porowska, J. Analysis of Cosmetic Products Containing Serratula coronata Herb Extract. Cosmetics 2023, 10, 18. https://doi.org/10.3390/cosmetics10010018
Kroma A, Feliczak-Guzik A, Pawlaczyk M, Osmałek T, Urbańska M, Micek I, Nawrot J, Gornowicz-Porowska J. Analysis of Cosmetic Products Containing Serratula coronata Herb Extract. Cosmetics. 2023; 10(1):18. https://doi.org/10.3390/cosmetics10010018
Chicago/Turabian StyleKroma, Anna, Agnieszka Feliczak-Guzik, Mariola Pawlaczyk, Tomasz Osmałek, Maria Urbańska, Iwona Micek, Joanna Nawrot, and Justyna Gornowicz-Porowska. 2023. "Analysis of Cosmetic Products Containing Serratula coronata Herb Extract" Cosmetics 10, no. 1: 18. https://doi.org/10.3390/cosmetics10010018
APA StyleKroma, A., Feliczak-Guzik, A., Pawlaczyk, M., Osmałek, T., Urbańska, M., Micek, I., Nawrot, J., & Gornowicz-Porowska, J. (2023). Analysis of Cosmetic Products Containing Serratula coronata Herb Extract. Cosmetics, 10(1), 18. https://doi.org/10.3390/cosmetics10010018