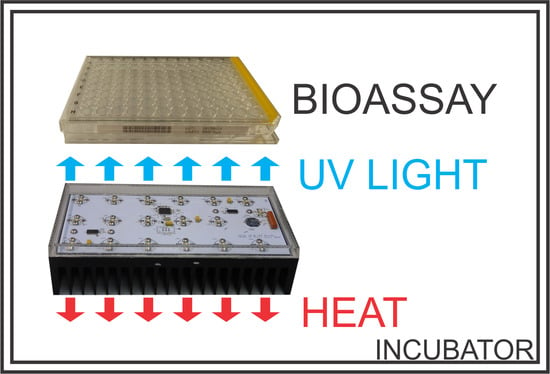
Compact UV LED Lamp with Low Heat Emissions for Biological Research Applications
Abstract
:1. Introduction
2. UV Lamp Design
2.1. UV Lamp Requirements and Optical Design
2.2. Electrical Design
2.3. Mechanical Design
3. Materials and Methods
3.1. Irradiance and Homogeneity
3.2. Spectrum and Transparency
3.3. Heat Dissipation
3.4. Cell Culture Tests
4. Results and Discussion
4.1. Simulation Results
4.2. Irradiance Homogeneity
4.3. Spectrum
4.4. Heat Dissipation
4.5. Cell Culture Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Emri, G.; Paragh, G.; Tósaki, Á.; Janka, E.; Kollár, S.; Hegedűs, C.; Gellén, E.; Horkay, I.; Koncz, G.; Remenyik, É. Ultraviolet radiation-mediated development of cutaneous melanoma: An update. J. Photochem. Photobiol. B Biol. 2018, 185, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Peng, C.; Jiang, H.; Zhu, Q.; Wang, W. Direct and Indirect Effects of UV-B Exposure on Litter Decomposition: A Meta-Analysis. PLoS ONE 2013, 8, e68858. [Google Scholar] [CrossRef] [PubMed]
- Maisels, M.J.; McDonagh, A.F. Phototherapy for Neonatal Jaundice. N. Engl. J. Med. 2008, 358, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Meesters, Y.; Duijzer, W.B.; Hommes, V. The effects of low-intensity narrow-band blue-light treatment compared to bright white-light treatment in seasonal affective disorder. J. Affect. Disord. 2018, 232, 48–51. [Google Scholar] [CrossRef]
- Holick, M.F. Ultraviolet B Radiation: The Vitamin D Connection. In Ultraviolet Light in Human Health, Diseases and Environment. Advances in Experimental Medicine and Biology; Ahmad, S., Ed.; Springer: Cham, Switzerland, 2017; Volume 996, pp. 137–154. ISBN 978-3-319-56017-5. [Google Scholar]
- Wang, Y.; Gutierrez-Herrera, E.; Ortega-Martinez, A.; Anderson, R.R.; Franco, W. UV fluorescence excitation imaging of healing of wounds in skin: Evaluation of wound closure in organ culture model. Lasers Surg. Med. 2016, 48, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lommerts, J.E.; Uitentuis, S.E.; Bekkenk, M.W.; de Rie, M.A.; Wolkerstorfer, A. The role of phototherapy in the surgical treatment of vitiligo: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Rodríguez, M.; Álvarez-Fuente, E.; Bustamante-Munguira, J.; Poves-Alvarez, R.; Fierro, I.; Gómez-Sánchez, E.; Gómez-Pesquera, E.; Lorenzo-López, M.; Eiros, J.M.; Álvarez, F.J.; et al. Impact of an ultraviolet air sterilizer on cardiac surgery patients, a randomized clinical trial. Med. Clin. 2018, 151, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sarkany, R.P.E. Ultraviolet Radiation and the Skin. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 469–482. [Google Scholar]
- Wischermann, K.; Boukamp, P.; Schmezer, P. Improved alkaline comet assay protocol for adherent HaCaT keratinocytes to study UVA-induced DNA damage. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 630, 122–128. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, I.; O’Brien, N. Modulation of UVA light-induced oxidative stress by β-carotene, lutein and astaxanthin in cultured fibroblasts. J. Dermatol. Sci. 1998, 16, 226–230. [Google Scholar] [CrossRef]
- Kyadarkunte, A.Y.; Patole, M.S.; Pokharkar, V.B. Cellular interactions and photoprotective effects of idebenone-loaded nanostructured lipid carriers stabilized using PEG-free surfactant. Int. J. Pharm. 2015, 479, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, H.; Kim, K.; Lim, K.-M.; Kim, J.; Jho, E.; Oh, E.-S. D-tyrosine negatively regulates melanin synthesis by competitively inhibiting tyrosinase activity. Pigment Cell Melanoma Res. 2018, 31, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, Y.; Huang, Y.; Lim, H.; Jang, K.; Kim, D.; Lim, C.-J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Park, S.L.; de la Vega, M.R.; Zhang, D.D.; Wondrak, G.T. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic. Biol. Med. 2015, 89, 690–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buechner, N.; Schroeder, P.; Jakob, S.; Kunze, K.; Maresch, T.; Calles, C.; Krutmann, J.; Haendeler, J. Changes of MMP-1 and collagen type Iα1 by UVA, UVB and IRA are differentially regulated by Trx-1. Exp. Gerontol. 2008, 43, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, N.; Andrieu-Abadie, N.; Tauler, R.; Bedia, C. Untargeted lipidomic analysis of primary human epidermal melanocytes acutely and chronically exposed to UV radiation. Mol. Omics 2018, 14, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Philips Lighting Philips TL 40W/12 RS SLV/25 Product Information. Available online: http://www.lighting.philips.com/main/prof/lamps/special-lamps/medical-lamps/medical-therapy-uvb-broad-band/uvb-broadband-tl/928011301201_EU/product (accessed on 10 July 2018).
- Dupuis, R.D.; Krames, M.R. History, Development, and Applications of High-Brightness Visible Light-Emitting Diodes. J. Light. Technol. 2008, 26, 1154–1171. [Google Scholar] [CrossRef]
- Hadis, M.A.; Cooper, P.R.; Milward, M.R.; Gorecki, P.C.; Tarte, E.; Churm, J.; Palin, W.M. Development and application of LED arrays for use in phototherapy research. J. Biophotonics 2017, 10, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, J.; Shin, J.H.; Lee, H.E.; Kang, I.-S.; Gwak, K.; Kim, D.-S.; Kim, D.; Lee, K.J. Optogenetic control of body movements via flexible vertical light-emitting diodes on brain surface. Nano Energy 2018, 44, 447–455. [Google Scholar] [CrossRef]
- Lee, H.E.; Lee, S.H.; Jeong, M.; Shin, J.H.; Ahn, Y.; Kim, D.; Oh, S.H.; Yun, S.H.; Lee, K.J. Trichogenic Photostimulation Using Monolithic Flexible Vertical AlGaInP Light-Emitting Diodes. ACS Nano 2018, 12, 9587–9595. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Choi, J.; Lee, S.H.; Jeong, M.; Shin, J.H.; Joe, D.J.; Kim, D.; Kim, C.W.; Park, J.H.; Lee, J.H.; et al. Monolithic Flexible Vertical GaN Light-Emitting Diodes for a Transparent Wireless Brain Optical Stimulator. Adv. Mater. 2018, 30, 1800649. [Google Scholar] [CrossRef] [PubMed]
- Matafonova, G.; Batoev, V. Recent advances in application of UV light-emitting diodes for degrading organic pollutants in water through advanced oxidation processes: A review. Water Res. 2018, 132, 177–189. [Google Scholar] [CrossRef]
- Cree Inc. Cree First to Break 300 Lumens-Per-Watt Barrier. Available online: http://www.cree.com/News-and-Events/Cree-News/Press-Releases/2014/March/300LPW-LED-barrier (accessed on 10 July 2018).
- Vilber BIO-SUN. Available online: http://www.vilber.de/en/products/uv-instruments/uv-irradiation-systems/bio-sun/ (accessed on 12 December 2018).
- Pirc, M.; Topič, M. Light Intensity and Homogeneity of A LED Based Portable UV Light Source For Medical Research. In Proceedings of the 53rd International Conference on Microelectronics, Devices and Materials, Ljubljana, Slovenia, 4–6 October 2017; Bernik, S., Topič, M., Malič, B., Eds.; MIDEM: Ljubljana, Slovenia, 2017; pp. 71–75. [Google Scholar]
- Lite-on Technology Corp. UV-A LED LTPL-C034UVH365. Available online: http://optoelectronics.liteon.com/%230%23/Led/LED-Component/Detail/908?param4=18 (accessed on 10 July 2018).
- European Commission Health & Consumer Protection Directorate-General. Opinion on Biological Effects of Ultraviolet Radiation Relevant to Health with Particular Reference to Sunbeds for Cosmetic Purposes; European Commission Health & Consumer Protection Directorate-General: Brussel, Belgium, 2006. [Google Scholar]
- Pirc, M.; Caserman, S. Comapct UV LED Lamp Measurement and Simulation Data. Available online: https://data.mendeley.com/datasets/wbhpcz67vn/1 (accessed on 20 March 2019).
- Kheyrandish, A.; Taghipour, F.; Mohseni, M. UV-LED radiation modeling and its applications in UV dose determination for water treatment. J. Photochem. Photobiol. A Chem. 2018, 352, 113–121. [Google Scholar] [CrossRef]
- Vilber VL-6LC. Available online: http://www.vilber.de/en/products/uv-instruments/uv-lamps/filtered-uv-lamps/vl-6lc/ (accessed on 17 December 2018).
- Khalil, C.; Shebaby, W. UVB damage onset and progression 24 h post exposure in human-derived skin cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Milleron, R.S.; Bratton, S.B. Heat Shock Induces Apoptosis Independently of Any Known Initiator Caspase-activating Complex. J. Biol. Chem. 2006, 281, 16991–17000. [Google Scholar] [CrossRef] [PubMed] [Green Version]

















| Column | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Row | |||||||
| 1 | 100 | 75 | 77 | 77 | 75 | 100 | |
| 2 | 75 | 55 | 56 | 56 | 55 | 75 | |
| 3 | 100 | 75 | 77 | 77 | 75 | 100 | |
| Column | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Row | |||||||
| 1 | 100.0 | 76.2 | 74.6 | 77.8 | 74.6 | 100.0 | |
| 2 | 77.8 | 55.6 | 58.7 | 55.6 | 60.3 | 79.4 | |
| 3 | 98.4 | 77.8 | 74.6 | 76.2 | 73.0 | 93.7 | |
| Column | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| Row | |||||||
| 1 | 90.5 | 84.1 | 82.5 | 79.4 | 79.4 | 98.4 | |
| 2 | 79.4 | 65.1 | 71.4 | 74.6 | 63.5 | 77.8 | |
| 3 | 100.0 | 79.4 | 100.0 | 81.0 | 82.5 | 96.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirc, M.; Caserman, S.; Ferk, P.; Topič, M. Compact UV LED Lamp with Low Heat Emissions for Biological Research Applications. Electronics 2019, 8, 343. https://doi.org/10.3390/electronics8030343
Pirc M, Caserman S, Ferk P, Topič M. Compact UV LED Lamp with Low Heat Emissions for Biological Research Applications. Electronics. 2019; 8(3):343. https://doi.org/10.3390/electronics8030343
Chicago/Turabian StylePirc, Matija, Simon Caserman, Polonca Ferk, and Marko Topič. 2019. "Compact UV LED Lamp with Low Heat Emissions for Biological Research Applications" Electronics 8, no. 3: 343. https://doi.org/10.3390/electronics8030343
APA StylePirc, M., Caserman, S., Ferk, P., & Topič, M. (2019). Compact UV LED Lamp with Low Heat Emissions for Biological Research Applications. Electronics, 8(3), 343. https://doi.org/10.3390/electronics8030343