Altered Levels of Desaturation and ω-6 Fatty Acids in Breast Cancer Patients’ Red Blood Cell Membranes
Abstract
:1. Introduction
2. Results
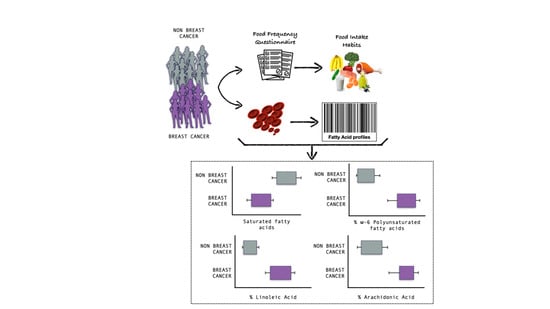
2.1. Population Characteristics and Dietary Intake
2.2. Red Blood Cell Membrane Fatty Acid Profile
3. Discussion
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Nutritional Status and Dietary Intake
4.3. Red Blood Cell Membrane Fatty Acid Profile Analysis
4.4. Red Blood Cell Membrane Fatty Acid Cluster
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, B.W.; Wild, C. World Cancer Report; International Agency for Research on Cancer: Lyon, France; World Health Organization: Geneva, Switzerland, 2014; p. XIV. 630p. [Google Scholar]
- Casares, D.; Escriba, P.V.; Rossello, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, L.T.; Chan, K.G.; Pusparajah, P.; Lee, W.L.; Chuah, L.H.; Khan, T.M.; Lee, L.H.; Goh, B.H. Targeting Membrane Lipid a Potential Cancer Cure? Front. Pharmacol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Ten Hagen, T.L. Cell Membrane Modulation as Adjuvant in Cancer Therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escriba, P.V.; Gonzalez-Ros, J.M.; Goni, F.M.; Kinnunen, P.K.; Vigh, L.; Sanchez-Magraner, L.; Fernandez, A.M.; Busquets, X.; Horvath, I.; Barcelo-Coblijn, G. Membranes: A Meeting Point for Lipids, Proteins and Therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, N.R.; Kim, E.; Fan, Y.Y.; Chapkin, R.S. Omega-3 Fatty Acids, Membrane Remodeling and Cancer Prevention. Mol. Asp. Med. 2018, 64, 79–91. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 Polyunsaturated Fatty Acids: From Biochemistry to Clinical Implications in Cardiovascular Prevention. Biochem. Pharm. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Ferreri, C.; Sansone, A.; Ferreri, R.; Amézaga, J.; Tueros, I. Fatty Acids and Membrane Lipidomics in Oncology: A Cross-Road of Nutritional, Signaling and Metabolic Pathways. Metabolites 2020, 10, 345. [Google Scholar] [CrossRef]
- Hendrich, A.B.; Michalak, K. Lipids as a Target for Drugs Modulating Multidrug Resistance of Cancer Cells. Curr. Drug Targets 2003, 4, 23–30. [Google Scholar] [CrossRef]
- Igal, R.A. Stearoyl CoA Desaturase-1: New Insights into a Central Regulator of Cancer Metabolism. Biochim. Biophys. Acta 2016, 1861, 1865–1880. [Google Scholar] [CrossRef]
- Eytan, G.D.; Regev, R.; Oren, G.; Assaraf, Y.G. The Role of Passive Transbilayer Drug Movement in Multidrug Resistance and Its Modulation. J. Biol. Chem. 1996, 271, 12897–12902. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary Long-Chain n-3 Fatty Acids for the Prevention of Cancer: A Review of Potential Mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Haubner, R.; Wurtele, G.; Hull, E.; Spiegelhalder, B.; Bartsch, H. Olives and Olive Oil in Cancer Prevention. Eur. J. Cancer Prev. 2004, 13, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Casto, B.C.; Harris, Z.M. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J. Clin. Oncol. 2014, 5, 677–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lands, B. Omega-3 PUFAs Lower the Propensity for Arachidonic Acid Cascade Overreactions. Biomed Res. Int. 2015, 2015, 285135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schley, P.D.; Brindley, D.N.; Field, C.J. (n-3) PUFA Alter Raft Lipid Composition and Decrease Epidermal Growth Factor Receptor Levels in Lipid Rafts of Human Breast Cancer Cells. J. Nutr. 2007, 137, 548–553. [Google Scholar] [CrossRef]
- Sanders, T.A. Fat and fatty acid intake and metabolic effects in the human body. Ann. Nutr. Metab. 2009, 55, 162–172. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; pp. 78–110. [Google Scholar]
- Asgari, Y.; Zabihinpour, Z.; Salehzadeh-Yazdi, A.; Schreiber, F.; Masoudi-Nejad, A. Alterations in Cancer Cell Metabolism: The Warburg Effect and Metabolic Adaptation. Genomics 2015, 105, 275–281. [Google Scholar] [CrossRef]
- Kato, Y.; Maeda, T.; Suzuki, A.; Baba, Y. Cancer Metabolism: New Insights into Classic Characteristics. Jpn. Dent. Sci. Rev. 2018, 54, 8–21. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid Metabolism and Carcinogenesis, Cancer Development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; De Stanchina, E.; Mathew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-Transformed Cells Support Growth by Scavenging Unsaturated Fatty Acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acar, N.; Berdeaux, O.; Grégoire, S.; Cabaret, S.; Martine, L.; Gain, P.; Thuret, G.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L. Lipid Composition of the Human Eye: Are Red Blood Cells a Good Mirror of Retinal and Optic Nerve Fatty Acids? PLoS ONE 2012, 7, e35102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dushianthan, A.; Cusack, R.; Koster, G.; Grocott, M.P.W.; Postle, A.D. Insight into Erythrocyte Phospholipid Molecular Flux in Healthy Humans and in Patients with Acute Respiratory Distress Syndrome. PLoS ONE 2019, 14, e0221595. [Google Scholar] [CrossRef]
- Ferreri, C.; Chatgilialoglu, C. Role of Fatty Acid-Based Functional Lipidomics in the Development of Molecular Diagnostic Tools. Expert Rev. Mol. Diagn. 2012, 12, 767–780. [Google Scholar] [CrossRef]
- Amezaga, J.; Arranz, S.; Urruticoechea, A.; Ugartemendia, G.; Larraioz, A.; Louka, M.; Uriarte, M.; Ferreri, C.; Tueros, I. Altered Red Blood Cell Membrane Fatty Acid Profile in Cancer Patients. Nutrients 2018, 10, 1853. [Google Scholar] [CrossRef] [Green Version]
- Pala, V.; Krogh, V.; Muti, P.; Chajes, V.; Riboli, E.; Micheli, A.; Saadatian, M.; Sieri, S.; Berrino, F. Erythrocyte Membrane Fatty Acids and Subsequent Breast Cancer: A Prospective Italian Study. J. Natl. Cancer Inst. 2001, 93, 1088–1095. [Google Scholar] [CrossRef]
- Merino Salvador, M.; Gomez de Cedron, M.; Moreno Rubio, J.; Falagan Martinez, S.; Sanchez Martinez, R.; Casado, E.; Ramirez de Molina, A.; Sereno, M. Lipid Metabolism and Lung Cancer. Crit. Rev. Oncol. Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef]
- Peck, B.; Schug, Z.T.; Zhang, Q.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of Fatty Acid Desaturation Is Detrimental to Cancer Cell Survival in Metabolically Compromised Environments. Cancer Metab. 2016, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Fan, X.X.; He, J.; Pan, H.; Li, R.Z.; Huang, L.; Jiang, Z.; Yao, X.J.; Liu, L.; Leung, E.L.; et al. SCD1 Is Associated with Tumor Promotion, Late Stage and Poor Survival in Lung Adenocarcinoma. Oncotarget 2016, 7, 39970–39979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeberle, A.; Loser, K.; Thurmer, M. Stearoyl-CoA Desaturase-1 and Adaptive Stress Signaling. Biochim. Biophys. Acta 2016, 1861, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Scanferlato, R.; Bortolotti, M.; Sansone, A.; Chatgilialoglu, C.; Polito, L.; De Spirito, M.; Maulucci, G.; Bolognesi, A.; Ferreri, C. Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borin, T.F.; Angara, K.; Rashid, M.H.; Achyut, B.R.; Arbab, A.S. Arachidonic Acid Metabolite as a Novel Therapeutic Target in Breast Cancer Metastasis. Int. J. Mol. Sci. 2017, 18, 2661. [Google Scholar] [CrossRef] [Green Version]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of Inflammation in Cancer by Eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Zanoaga, O.; Jurj, A.; Raduly, L.; Cojocneanu-Petric, R.; Fuentes-Mattei, E.; Wu, O.; Braicu, C.; Gherman, C.D.; Berindan-Neagoe, I. Implications of Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids in Breast Cancer. Exp. Ther. Med. 2018, 15, 1167–1176. [Google Scholar] [CrossRef]
- Fernandez-Ballart, J.D.; Pinol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Martin-Moreno, J.M. Relative Validity of a Semi-Quantitative Food-Frequency Questionnaire in an Elderly Mediterranean Population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Ortega, R.M.; Lopez-Sobaler, A.M.; Andres, P.; Requejo, A.M.; Aparicio, A.; Molinero, L.M. DIAL Software for Assessing Diets and Food Calculations, 3.5.0.3; Departament of Nutrition (UCM) & Alce Ingenieria, S.L.: Madrid, Spain, 2017. [Google Scholar]
- Ghezzo, A.; Visconti, P.; Abruzzo, P.M.; Bolotta, A.; Ferreri, C.; Gobbi, G.; Malisardi, G.; Manfredini, S.; Marini, M.; Nanetti, L.; et al. Oxidative Stress and Erythrocyte Membrane Alterations in Children with Autism: Correlation with Clinical Features. PLoS ONE 2013, 8, e66418. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, G.; Ferreri, C.; Sansone, A.; Chatgilialoglu, C.; Marzetti, C.; Spyratou, E.; Georgakilas, A.G.; Marini, M.; Abruzzo, P.M.; Bolotta, A.; et al. High Predictive Values of RBC Membrane-Based Diagnostics by Biophotonics in an Integrated Approach for Autism Spectrum Disorders. Sci. Rep. 2017, 7, 9854. [Google Scholar] [CrossRef]
- Sansone, A.; Tolika, E.; Louka, M.; Sunda, V.; Deplano, S.; Melchiorre, M.; Anagnostopoulos, D.; Chatgilialoglu, C.; Formisano, C.; Di Micco, R.; et al. Hexadecenoic Fatty Acid Isomers in Human Blood Lipids and Their Relevance for the Interpretation of Lipidomic Profiles. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Van der Vegt, S.G.; Ruben, A.M.; Werre, J.M.; Palsma, D.M.; Verhoef, C.W.; De Gier, J.; Staal, G.E. Counterflow Centrifugation of Red Cell Populations: A Cell Age Related Separation Technique. Br. J. Haematol. 1985, 61, 393–403. [Google Scholar] [CrossRef]
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch Methods for Solid-Liquid-Liquid Extraction of Lipids from Microorganisms. Comprehension of Solvatation Mechanisms and towards Substitution with Alternative Solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatgilialoglu, C.; Ferreri, C. Nutrilipidomics: A Tool for Personalized Health. J. Glycom. Lipidom. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A New Risk Factor for Death from Coronary Heart Disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty Acid Composition of Membrane Bilayers: Importance of Diet Polyunsaturated Fat Balance. Biochim. Biophys. Acta 2012, 5, 18. [Google Scholar] [CrossRef] [Green Version]

| Characteristics/Intake | Total Nutrient/Food Intake | ||
|---|---|---|---|
| Control (n = 39) Mean ± SE | Cancer (n = 45) Mean ± SE | p-Value | |
| Population characteristics | |||
| HER2- | 29 (64.4 %) | ||
| HER2+ | 16 (35.6 %) | ||
| Age (years) | 43.08 ± 2.16 | 52.84 ± 1.40 | <0.001 |
| BMI (kg/m2) | 23.93 ± 0.44 | 24.20 ± 0.41 | 0.650 |
| Daily Energy and Nutrient Intake | |||
| Calories (Kcal/day) | 2068.97 ± 122.39 | 2539.24 ± 81.71 | 0.002 |
| Carbohydrates (% of energy/day) | 38.13 ± 1.03 | 36.09 ± 0.81 | 0.116 |
| Simple sugars (g/day) | 108.54 ± 8.24 | 101.68 ± 4.89 | 0.477 |
| Protein (% of energy/day) * | 17.54 ± 0.46 | 16.67 ± 0.14 | 0.221 |
| Fiber (g/day) * | 31.49 ± 2.34 | 34.70 ± 2.15 | 0.099 |
| Alcohol (g/day) * | 4.14 ± 0.93 | 11.25 ± 2.52 | 0.134 |
| Fat (% of energy/day) | 39.67 ± 0.96 | 41.42 ± 0.80 | 0.162 |
| SFA (% of energy/day) | 11.00 ± 0.35 | 10.71 ± 0.29 | 0.519 |
| C14:0 (g/day) | 2.00 ± 0.17 | 2.12 ± 0.17 | 0.661 |
| C16:0 (g/day) | 13.19 ± 0.84 | 17.51 ± 0.74 | <0.001 |
| C18:0 (g/day) | 5.66 ± 0.40 | 7.31 ± 0.36 | 0.001 |
| MUFA (% of energy/day) | 18.60 ± 0.63 | 20.05 ± 0.55 | 0.085 |
| C16:1 (g/day) | 1.19 ± 0.08 | 1.59 ± 0.06 | 0.001 |
| C18:1 (g/day) * | 40.48 ± 2.86 | 52.77 ± 1.90 | <0.001 |
| PUFA (% of energy/day) | 6.79 ± 0.33 | 6.81 ± 0.25 | 0.970 |
| W3 (% of energy/day) | 1.15 ± 0.06 | 1.16 ± 0.05 | 0.850 |
| C18:3 (g/day) | 2.04 ± 0.18 | 2.17 ± 0.11 | 0.154 |
| C20:5 (g/day) | 0.17 ± 0.02 | 0.32 ± 0.04 | 0.001 |
| C22:5 (g/day) * | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.002 |
| C22:6 (g/day) | 0.36 ± 0.04 | 0.62 ± 0.05 | <0.001 |
| W6 (% of energy/day) | 5.47 ± 0.28 | 5.45 ± 0.22 | 0.947 |
| C18:2 (g/day) | 12.71 ± 1.11 | 15.26 ± 0.84 | 0.015 |
| C20:4 (g/day) | 0.14 ± 0.01 | 0.18 ± 0.01 | 0.001 |
| Food Groups (g/day) | |||
| Oily fish | 22.74 ± 2.84 | 27.05 ± 3.41 | 0.069 |
| Lean fish | 28.49 ± 3.01 | 50.06 ± 4.93 | <0.001 |
| Shellfish | 16.82 ± 4.27 | 9.43 ± 1.29 | 0.069 |
| Olive oil | 31.72 ± 2.58 | 45.26 ± 2.18 | <0.001 |
| Sunflower oil | 0.30 ± 0.11 | 2.36 ± 0.82 | 0.037 |
| Nuts | 14.42 ± 2.47 | 13.81 ± 2.02 | 0.522 |
| Fruit | 302.08 ± 33.83 | 310.85 ± 26.05 | 0.263 |
| Vegetables | 315.09 ± 30.35 | 306.27 ± 14.90 | 0.422 |
| Dairy products | 404.47 ± 35.11 | 358.14 ± 37.28 | 0.290 |
| Eggs | 20.04 ± 2.55 | 31.90 ± 1.69 | 0.002 |
| Red meat | 35.96 ± 4.44 | 30.33 ± 2.89 | <0.001 |
| Cold meat | 15.61 ± 2.00 | 28.81 ± 2.41 | <0.001 |
| RBC Membrane FA (% rel) | Unadjusted Control | Unadjusted Cancer | p-Value | Adjusted Control | Adjusted Cancer | p-Value | Difference (%) |
|---|---|---|---|---|---|---|---|
| (n = 39) | (n = 45) | (n = 39) | (n = 45) | ||||
| Saturated Fatty Acids | |||||||
| Palmitic acid (16:0) | 24.5 ± 0.4 | 22.6 ± 0.2 | <0.001 | 24.5 ± 0.3 | 22.6 ± 0.3 | <0.001 | −7.5 |
| Stearic acid (18:0) | 19.8 ± 0.3 | 18.2 ± 0.2 | <0.001 | 19.9 ± 0.3 | 18.1 ± 0.3 | <0.001 | −9.2 |
| Monounsaturated Fatty Acids | |||||||
| Palmitoleic acid (16:1; 9c) * | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.002 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.258 | −10.0 |
| OA (18:1; 9c) * | 16.4 ± 0.3 | 17.4 ± 0.2 | 0.005 | 16.7 ± 0.3 | 17.1 ± 0.2 | 0.283 | 2.6 |
| Cis-vaccenic acid (18:1; 11c *) | 1.2 ± 0.0 | 1.4 ± 0.0 | <0.001 | 1.2 ± 0.0 | 1.5 ± 0.0 | <0.001 | 25.0 |
| Polyunsaturated Acid | |||||||
| LA (18:2 ω-6) | 12.1 ± 0.2 | 12.8 ± 0.2 | 0.035 | 11.8 ± 0.3 | 13.1 ± 0.2 | 0.001 | 11.1 |
| DGLA (20:3 ω-6) * | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.374 | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.186 | 11.1 |
| AA (20:4 ω-6) * | 17.3 ± 0.3 | 18.2 ± 0.2 | 0.017 | 17.1 ± 0.3 | 18.4 ± 0.3 | 0.006 | 7.4 |
| EPA (20:5 ω-3) * | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.076 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.971 | 0.0 |
| DHA (22:6 ω-3) | 5.4 ± 0.2 | 6.0 ± 0.2 | 0.042 | 5.6 ± 0.2 | 5.8 ± 0.2 | 0.588 | 3.2 |
| Trans Fatty Acids | |||||||
| Trans 18:1 * | 0.2 ± 0.0 | 0.1 ± 0.0 | <0.001 | 0.2 ± 0.0 | 0.1 ± 0.0 | <0.001 | −63.2 |
| Trans 20:4 * | 0.2 ± 0.0 | 0.1 ± 0.0 | <0.001 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.002 | −44.4 |
| Total Fatty Acids | |||||||
| Total SFA | 44.4 ± 0.6 | 40.8 ± 0.2 | 0.009 | 44.4 ± 0.5 | 40.7 ± 0.4 | <0.001 | −8.3 |
| Total MUFA | 18.1 ± 0.2 | 19.2 ± 0.2 | 0.003 | 18.4 ± 0.3 | 19.0 ± 0.3 | 0.115 | 3.6 |
| Total PUFA | 37.4 ± 0.4 | 39.9 ± 0.3 | <0.001 | 37.1 ± 0.4 | 40.1 ± 0.3 | <0.001 | 7.9 |
| Total omega 6 | 31.3 ± 0.4 | 33.0 ± 0.3 | 0.002 | 30.7 ± 0.4 | 33.5 ± 0.4 | <0.001 | 9.0 |
| Total trans * | 0.4 ± 0.0 | 0.2 ± 0.0 | <0.001 | 0.4 ± 0.0 | 0.2 ± 0.0 | <0.001 | −48.6 |
| Fatty Acid Indexes—Ratios | |||||||
| SFA/MUFA | 2.5 ± 0.1 | 2.1 ± 0.0 | 0.002 | 2.5 ± 0.1 | 2.2 ± 0.1 | 0.001 | −12.2 |
| Unsaturation index | 153.3 ± 1.8 | 164.1 ± 1.1 | <0.001 | 153.7 ± 1.6 | 163.7 ± 1.4 | <0.001 | 6.5 |
| Peroxidation index | 133.2 ± 2.0 | 143.5 ± 1.6 | <0.001 | 134.3 ± 2.0 | 142.5 ± 1.8 | 0.007 | 6.5 |
| Inflammatory risk index * | 5.8± 0.5 | 5.1 ± 0.2 | 0.625 | 5.3 ± 0.4 | 5.5 ± 0.4 | 0.747 | 3.6 |
| PUFA balance | 16.5 ± 0.7 | 17.3 ± 0.6 | 0.337 | 17.4 ± 0.7 | 16.5 ± 0.6 | 0.390 | −5.1 |
| n-3 cardiovascular risk index | 6.1 ± 0.3 | 6.9 ± 0.2 | 0.036 | 6.5 ± 0.3 | 6.6 ± 0.3 | 0.653 | 2.8 |
| Enzymatic Indexes | |||||||
| SCD1 18:0/18:1 * | 1.2 ± 0.0 | 1.1 ± 0.0 | <0.001 | 1.2 ± 0.0 | 1.1 ± 0.0 | 0.003 | −13.1 |
| Δ6D+ELO 20:3/18:2 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.904 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.893 | 1.0 |
| Δ5D 20:4/20:3 | 9.8 ± 0.4 | 9.92 ± 0.4 | 0.796 | 9.9 ± 0.5 | 9.8 ± 0.5 | 0.889 | −1.1 |
| SCD1 16:0/16:1 * | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.037 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.524 | 7.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amézaga, J.; Ugartemendia, G.; Larraioz, A.; Bretaña, N.; Iruretagoyena, A.; Camba, J.; Urruticoechea, A.; Ferreri, C.; Tueros, I. Altered Levels of Desaturation and ω-6 Fatty Acids in Breast Cancer Patients’ Red Blood Cell Membranes. Metabolites 2020, 10, 469. https://doi.org/10.3390/metabo10110469
Amézaga J, Ugartemendia G, Larraioz A, Bretaña N, Iruretagoyena A, Camba J, Urruticoechea A, Ferreri C, Tueros I. Altered Levels of Desaturation and ω-6 Fatty Acids in Breast Cancer Patients’ Red Blood Cell Membranes. Metabolites. 2020; 10(11):469. https://doi.org/10.3390/metabo10110469
Chicago/Turabian StyleAmézaga, Javier, Gurutze Ugartemendia, Aitziber Larraioz, Nerea Bretaña, Aizpea Iruretagoyena, Joana Camba, Ander Urruticoechea, Carla Ferreri, and Itziar Tueros. 2020. "Altered Levels of Desaturation and ω-6 Fatty Acids in Breast Cancer Patients’ Red Blood Cell Membranes" Metabolites 10, no. 11: 469. https://doi.org/10.3390/metabo10110469
APA StyleAmézaga, J., Ugartemendia, G., Larraioz, A., Bretaña, N., Iruretagoyena, A., Camba, J., Urruticoechea, A., Ferreri, C., & Tueros, I. (2020). Altered Levels of Desaturation and ω-6 Fatty Acids in Breast Cancer Patients’ Red Blood Cell Membranes. Metabolites, 10(11), 469. https://doi.org/10.3390/metabo10110469