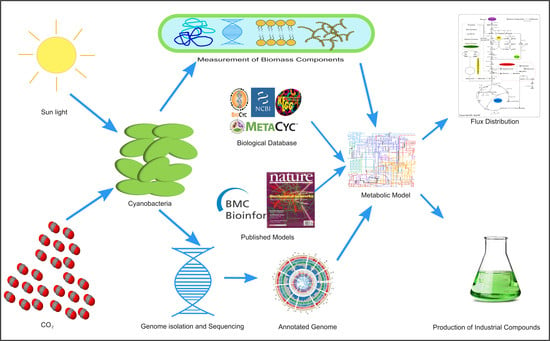
Biochemical Characteristics and a Genome-Scale Metabolic Model of an Indian Euryhaline Cyanobacterium with High Polyglucan Content
Abstract
:1. Introduction
2. Results
2.1. Growth and Total Carbohydrate Content of Synechococcus sp. BDU 130192 and Synechococcus sp. PCC 7002
2.2. Structural analysis of Synechococcus sp. BDU 130192 and PCC 7002
2.3. Oxygen Evolution Rate and Dark Respiration Rate
2.4. Glycogen Synthesis Genes Transcript Levels
2.5. Biomass Composition of Synechococcus sp. BDU 130192 and Its Comparison to That of Synechococcus sp. PCC 7002
2.6. Phylogenetic Analysis of Synechococcus sp. BDU 130192
2.7. Gap-Filling and General Properties of the Model
2.7.1. Analysis of Gap-Filling Reactions
2.7.2. General Properties of the Model
2.8. Model Simulations
2.9. Reaction Deletion Analysis to Identify Essential Reactions
2.10. Detailing Metabolism under Photoautotrophic Condition and the Maximum Theoretical Yields of Native and Heterologous Compounds
3. Discussion
4. Materials and Methods
4.1. Culture Conditions
4.2. Microscopic Analysis of Cyanobacterial Cells Using Scanning Electron Microscopy (SEM)
4.3. Measurement of Oxygen Evolution and Dark Respiration Rates
4.4. Estimation of Biomass Composition
4.5. RNA Extraction, cDNA Synthesis and Transcriptional Analysis by RT-PCR
4.6. Phylogenetic Analysis
4.7. Reconstruction of the Genome-Scale Metabolic Model
4.7.1. Draft Model
4.7.2. Formation of the Biomass Equation, Biomass Formula and Biomass Degree of Reduction
4.7.3. Gap Filling and Model Refining
4.7.4. Energy Requirements
4.8. Model Simulations for Autotrophic Condition and Reaction Essentiality Analysis
4.9. Production of Industrially-Relevant Bio-Products
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New Applications of Synthetic Biology Tools for Cyanobacterial Metabolic Engineering. Front. Bioeng. Biotechnol. 2019, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhang, H.; Zhang, Y.; Li, Y.; Ma, Y. Designing and creating a modularized synthetic pathway in cyanobacterium Synechocystis enables production of acetone from carbon dioxide. Metab. Eng. 2012, 14, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Nozzi, N.E.; Oliver, J.W.K.; Atsumi, S. Cyanobacteria as a Platform for Biofuel Production. Front. Bioeng. Biotechnol. 2013, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Narainsamy, K.; Marteyn, B.; Sakr, S. Genomics of the Pleïotropic Glutathione System in Cyanobacteria. Adv. Bot. Res. 2013, 65, 157–188. [Google Scholar]
- Porter, R.D. Transformation in Cyanobacteria. CRC Crit. Rev. Microbiol. 1986, 13, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Gleick, P.H.; Palaniappan, M. Peak water limits to freshwater withdrawal and use. Proc. Natl. Acad. Sci. USA 2010, 107, 11155–11162. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Carbohydrate-enriched cyanobacterial biomass as feedstock for bio-methane production through anaerobic digestion. Fuel 2013, 111, 872–879. [Google Scholar] [CrossRef]
- Hays, S.G.; Ducat, D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth. Res. 2015, 123, 285–295. [Google Scholar] [CrossRef]
- Möllers, K.; Cannella, D.; Jørgensen, H.; Frigaard, N.-U. Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol. Biofuels 2014, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-H.; Spalding, M.H.; Jane, J. Characterization of cyanobacterial glycogen isolated from the wild type and from a mutant lacking of branching enzyme. Carbohydr. Res. 2002, 337, 2195–2203. [Google Scholar] [CrossRef]
- Pathania, R.; Ahmad, A.; Srivastava, S. Draft Genome Sequence of an Indian Marine Cyanobacterial Strain with Fast Growth and High Polyglucan Content. Genome Announc. 2017, 5, e01334-17. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Kim, M.K.; Kumaraswamy, G.K.; Agarwal, A.; Lun, D.S.; Dismukes, G.C. Flux balance analysis of photoautotrophic metabolism: Uncovering new biological details of subsystems involved in cyanobacterial photosynthesis. Biochim. Biophys. Acta Bioenergy 2017, 1858, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, R.; Edwards, J.S.; Doyle, F.J. Dynamic flux balance analysis of diauxic growth. Biophys. J. 2002, 83, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.A.; Palsson, B.O. Context-specific metabolic networks are consistent with experiments. PLoS Comput. Biol. 2008, 4, e1000082. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Reed, J.L. RELATCH: Relative optimality in metabolic networks explains robust metabolic and regulatory responses to perturbations. Genome Biol. 2012, 13, R78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- O’Brien, E.J.; Monk, J.M.; Palsson, B.O. Using Genome-scale Models to Predict Biological Capabilities. Cell 2015, 161, 971–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoop, H.; Zilliges, Y.; Lockau, W.; Steuer, R. The metabolic network of Synechocystis sp. PCC 6803: Systemic properties of autotrophic growth. Plant Physiol. 2010, 154, 410–422. [Google Scholar] [CrossRef] [Green Version]
- Nogales, J.; Gudmundsson, S.; Knight, E.M.; Palsson, B.O.; Thiele, I. Detailing the optimality of photosynthesis in cyanobacteria through systems biology analysis. Proc. Natl. Acad. Sci. USA 2012, 109, 2678–2683. [Google Scholar] [CrossRef] [Green Version]
- Baart, G.J.E.; Martens, D.E. Genome-Scale Metabolic Models: Reconstruction and Analysis; Humana Press: Totowa, NJ, USA, 2012; pp. 107–126. [Google Scholar]
- Broddrick, J.T.; Rubin, B.E.; Welkie, D.G.; Du, N.; Mih, N.; Diamond, S.; Lee, J.J.; Golden, S.S.; Palsson, B.O.; Atsumi, S.; et al. Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. USA 2016, 113, E8344–E8353. [Google Scholar] [CrossRef] [Green Version]
- Gomes de Oliveira Dal’Molin, C.; Quek, L.-E.; Palfreyman, R.W.; Nielsen, L.K. AlgaGEM—A genome-scale metabolic reconstruction of algae based on the Chlamydomonas reinhardtii genome. BMC Genomics 2011, 12, S5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, R.L.; Ghamsari, L.; Manichaikul, A.; Hom, E.F.Y.; Balaji, S.; Fu, W.; Shen, Y.; Hao, T.; Palsson, B.O.; Salehi-Ashtiani, K.; et al. Metabolic network reconstruction of Chlamydomonas offers insight into light-driven algal metabolism. Mol. Syst. Biol. 2014, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Chaplen, F.W.R.; Murthy, G.S. Genome scale metabolic reconstruction of Chlorella variabilis for exploring its metabolic potential for biofuels. Bioresour. Technol. 2016, 213, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, C.; Li, C.-T.; Huelsman, T.; Levering, J.; Zielinski, D.C.; McConnell, B.O.; Long, C.P.; Knoshaug, E.P.; Guarnieri, M.T.; Antoniewicz, M.R.; et al. Genome-Scale Metabolic Model for the Green Alga Chlorella vulgaris UTEX 395 Accurately Predicts Phenotypes under Autotrophic, Heterotrophic, and Mixotrophic Growth Conditions. Plant Physiol. 2016, 172, 589–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.R.; Ahmad, A.; Srivastava, S.; Jaffar Ali, B.M. Reconstruction and analysis of a genome-scale metabolic model of Nannochloropsis gaditana. Algal Res. 2017, 26, 354–364. [Google Scholar] [CrossRef]
- Loira, N.; Mendoza, S.; Paz Cortés, M.; Rojas, N.; Travisany, D.; Genova, A.D.; Gajardo, N.; Ehrenfeld, N.; Maass, A. Reconstruction of the microalga Nannochloropsis salina genome-scale metabolic model with applications to lipid production. BMC Syst. Biol. 2017, 11, 66. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Matsuo, S.; Hamada, H.; Matsuda, F.; Hanai, T. Metabolic engineering of Synechococcus elongatus PCC 7942 for improvement of 1,3-propanediol and glycerol production based on in silico simulation of metabolic flux distribution. Microb. Cell Fact. 2017, 16, 212. [Google Scholar] [CrossRef] [Green Version]
- Shirai, T.; Osanai, T.; Kondo, A. Designing intracellular metabolism for production of target compounds by introducing a heterologous metabolic reaction based on a Synechosystis sp. 6803 genome-scale model. Microb. Cell Fact. 2016, 15, 13. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Aikawa, S.; Kojima, Y.; Toya, Y.; Furusawa, C.; Kondo, A.; Shimizu, H. Construction of a Genome-Scale Metabolic Model of Arthrospira platensis NIES-39 and Metabolic Design for Cyanobacterial Bioproduction. PLoS ONE 2015, 10, e0144430. [Google Scholar] [CrossRef]
- Mueller, T.J.; Berla, B.M.; Pakrasi, H.B.; Maranas, C.D. Rapid construction of metabolic models for a family of Cyanobacteria using a multiple source annotation workflow. BMC Syst. Biol. 2013, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Karp, P.D.; Weaver, D.; Latendresse, M. How accurate is automated gap filling of metabolic models? BMC Syst. Biol. 2018, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Griese, M.; Lange, C.; Soppa, J. Ploidy in cyanobacteria. FEMS Microbiol. Lett. 2011, 323, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendry, J.I.; Prasannan, C.B.; Joshi, A.; Dasgupta, S.; Wangikar, P.P. Metabolic model of Synechococcus sp. PCC 7002: Prediction of flux distribution and network modification for enhanced biofuel production. Bioresour. Technol. 2016, 213, 190–197. [Google Scholar] [CrossRef]
- Vu, T.T.; Hill, E.A.; Kucek, L.A.; Konopka, A.E.; Beliaev, A.S.; Reed, J.L. Computational evaluation of Synechococcus sp. PCC 7002 metabolism for chemical production. Biotechnol. J. 2013, 8, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.E.; Bernstein, H.C.; Carlson, R.P. Stoichiometric network analysis of cyanobacterial acclimation to photosynthesis-associated stresses identifies heterotrophic niches. Processes 2017, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Becker, S.A.; Feist, A.M.; Mo, M.L.; Hannum, G.; Palsson, B.Ø.; Herrgard, M.J. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox. Nat. Protoc. 2007, 2, 727–738. [Google Scholar] [CrossRef]
- Poolman, M.G. ScrumPy: Metabolic modelling with Python. Syst. Biol. (Stevenage) 2006, 153, 375–378. [Google Scholar] [CrossRef]
- Gelius-Dietrich, G.; Desouki, A.; Fritzemeier, C.; Lercher, M.J. Sybil—Efficient constraint-based modelling in R. BMC Syst. Biol. 2013, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.J.; Reed, J.L. Identification of Functional Differences in Metabolic Networks Using Comparative Genomics and Constraint-Based Models. PLoS ONE 2012, 7, e34670. [Google Scholar] [CrossRef] [Green Version]
- Montagud, A.; Zelezniak, A.; Navarro, E.; de Córdoba, P.F.; Urchueguía, J.F.; Patil, K.R. Flux coupling and transcriptional regulation within the metabolic network of the photosynthetic bacterium Synechocystis sp. PCC6803. Biotechnol. J. 2011, 6, 330–342. [Google Scholar] [CrossRef]
- Zhang, S.; Bryant, D.A. The Tricarboxylic Acid Cycle in Cyanobacteria. Science 2011, 334, 1551–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, T.; Cai, Z.; Li, Y. From cyanochemicals to cyanofactories: A review and perspective. Microb. Cell Fact. 2016, 15, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; He, Q. Cyanobacteria as cell factories to produce plant secondary metabolites. Front. Bioeng. Biotechnol. 2015, 3, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, A.E.; Hunt, K.A.; Carlson, R.P. Measuring cellular biomass composition for computational biology applications. Processes 2018, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.A.; Liu, Z.; Rodionov, D.A.; Li, Z.; Bryant, D.A. Complementation of Cobalamin Auxotrophy in Synechococcus sp. Strain PCC 7002 and Validation of a Putative Cobalamin Riboswitch In Vivo. J. Bacteriol. 2016, 198, 2743–2752. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, N.T.; Riisgård, F.K.; Gunther, W.S.; Lønsmann Iversen, J.J. On-line estimation of O2 production, CO2 uptake, and growth kinetics of microalgal cultures in a gas-tight photobioreactor. Proc. J. Appl. Phycol. 2007, 19, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Pharkya, P.; Burgard, A.P.; Maranas, C.D. OptStrain: A computational framework for redesign of microbial production systems. Genome Res. 2004, 14, 2367–2376. [Google Scholar] [CrossRef] [Green Version]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Jaiswal, D.; Sengupta, A.; Sohoni, S.; Sengupta, S.; Phadnavis, A.G.; Pakrasi, H.B.; Wangikar, P.P. Genome Features and Biochemical Characteristics of a Robust, Fast Growing and Naturally Transformable Cyanobacterium Synechococcus elongatus PCC 11801 Isolated from India. Sci. Rep. 2018, 8, 16632. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.; Degnan, B.; Degnan, S.; Krömer, J.O. Determining the Biomass Composition of a Sponge Holobiont for Flux Analysis; Humana Press: New York, NY, USA, 2014; pp. 107–125. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Cesarone, C.F.; Bolognesi, C.; Santi, L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal. Biochem. 1979, 100, 188–197. [Google Scholar] [CrossRef]
- Fleck, A.; Munro, H.N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim. Biophys. Acta 1962, 55, 571–583. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, E.; Sicora, C.; Dragoş, N.; Drugă, B. Selection of proper reference genes for the cyanobacterium Synechococcus PCC 7002 using real-time quantitative PCR. FEMS Microbiol. Lett. 2014, 359, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Thiele, I.; Palsson, B. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010, 5, 93–121. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Placzek, S.; Schomburg, I.; Chang, A.; Jeske, L.; Ulbrich, M.; Tillack, J.; Schomburg, D. BRENDA in 2017: New perspectives and new tools in BRENDA. Nucleic Acids Res. 2017, 45, D380–D388. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Cheung, C.Y.M.; Poolman, M.G.; Hilbers, P.A.J.; van Riel, N.A.W. A genome-scale metabolic network reconstruction of tomato (Solanum lycopersicum L.) and its application to photorespiratory metabolism. Plant J. 2016, 85, 289–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, N.-R.; Lakshmanan, M.; Aggarwal, S.; Song, J.-W.; Karimi, I.A.; Lee, D.-Y.; Park, J.-B. Genome-scale metabolic network reconstruction and in silico flux analysis of the thermophilic bacterium Thermus thermophilus HB27. Microb. Cell Fact. 2014, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Poolman, M.G.; Kundu, S.; Shaw, R.; Fell, D.A. Metabolic trade-offs between biomass synthesis and photosynthate export at different light intensities in a genome-scale metabolic model of rice. Front. Plant. Sci. 2014, 5, 656. [Google Scholar] [CrossRef] [Green Version]







| S. No. | Components | BDU 130192 (mg/mg DCW) | PCC 7002 (mg/mg DCW) |
|---|---|---|---|
| 1. | Protein | 0.41 ± 0.010 # | 0.61 ± 0.0062 |
| 2. | Total Carbohydrates Glycogen | 0.52 ± 0.065 * 0.417 ± 0.036 * | 0.25 ± 0.013 0.180 ± 0.007 |
| 3. | Total Lipids | 0.0370 ± 0.0005 # | 0.046 ± 0.0017 |
| 4. | RNA | 0.0441 ± 0.0016 # | 0.069 ± 0.0025 |
| 5. | DNA | 0.0045 ± 0.00023 * | 0.0021 ± 0.00037 |
| 6. | Chlorophyll | 0.0049 ± 0.0006 # | 0.0154 ± 0.0012 |
| 7. | Carotenoids | 0.0035 ± 0.0002 | 0.0039 ± 0.0004 |
| 8. | Phycobiliproteins | 0.00031 ± 0.00004 # | 0.007 ± 0.0003 |
| Model Name | Species Name | No. of Genes | No. of Reaction | No. of Metabolites | No. of Active Reactions | No. of Essential Reactions | Reference |
|---|---|---|---|---|---|---|---|
| iSyn706 | Synechococcus sp. BDU 130192 | 706 | 908 | 900 | 502 | 450 | This Study |
| iSyp708 | Synechococcus sp. PCC 7002 | 705 | 647 | 622 | 322 | 277 | [35] |
| iSyp611 | Synechococcus sp. PCC 7002 | 611 | 589 | 579 | 344 | 297 | [40] |
| iJN678 | Synechocystis sp. PCC 6803 | 678 | 864 | 795 | 529 | 481 | [19] |
| iJB785 | Synechococcus elongatus PCC 7942 | 785 | 850 | 786 | - | - | [21] |
| iSyn811 | Synechocystis sp. PCC 6803 | 811 | 956 | 911 | - | - | [41] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.; Pathania, R.; Srivastava, S. Biochemical Characteristics and a Genome-Scale Metabolic Model of an Indian Euryhaline Cyanobacterium with High Polyglucan Content. Metabolites 2020, 10, 177. https://doi.org/10.3390/metabo10050177
Ahmad A, Pathania R, Srivastava S. Biochemical Characteristics and a Genome-Scale Metabolic Model of an Indian Euryhaline Cyanobacterium with High Polyglucan Content. Metabolites. 2020; 10(5):177. https://doi.org/10.3390/metabo10050177
Chicago/Turabian StyleAhmad, Ahmad, Ruchi Pathania, and Shireesh Srivastava. 2020. "Biochemical Characteristics and a Genome-Scale Metabolic Model of an Indian Euryhaline Cyanobacterium with High Polyglucan Content" Metabolites 10, no. 5: 177. https://doi.org/10.3390/metabo10050177
APA StyleAhmad, A., Pathania, R., & Srivastava, S. (2020). Biochemical Characteristics and a Genome-Scale Metabolic Model of an Indian Euryhaline Cyanobacterium with High Polyglucan Content. Metabolites, 10(5), 177. https://doi.org/10.3390/metabo10050177