Metabolic Signatures of 10 Processed and Non-processed Meat Products after In Vitro Digestion
Abstract
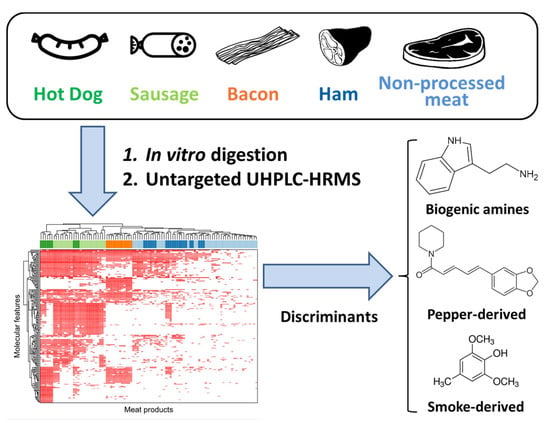
:1. Introduction
2. Results
2.1. Metabolite Profiles of Meat Products
2.2. Metabolites Significantly Elevated in Processed Meat Products
2.3. Metabolites Specific for Fermented Meat Products
3. Discussion
4. Materials and Methods
4.1. Reagents and Meat Products
4.2. In Vitro Digestion
4.3. Liquid Chromatography-Mass Spectrometry Analysis
4.4. Data Processing
4.5. Metabolite Annotation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Abbreviations
References
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinz, G.; Hautzinger, P. Meat Processing Technology for Small- to Medium-Scale Producers; RAP Publication: Bangkok, Thailand; Food and Agriculture Organizations of the United Nations: Rome, Italy; Regional Office for Asia and the Pacific: Bangkok, Thailand, 2007; ISBN 978-974-7946-99-4. [Google Scholar]
- Demeyer, D.; Mertens, B.; Smet, S.D.; Ulens, M. Mechanisms Linking Colorectal Cancer to the Consumption of (Processed) Red Meat: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2747–2766. [Google Scholar] [CrossRef] [Green Version]
- Kazerouni, N.; Sinha, R.; Hsu, C.H.; Greenberg, A.; Rothman, N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol. 2001, 39, 423–436. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Duedahl-Olesen, L.; Granby, K. Occurrence of volatile and non-volatile N-nitrosamines in processed meat products and the role of heat treatment. Food Control 2015, 48, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Steppeler, C.; Haugen, J.-E.; Rødbotten, R.; Kirkhus, B. Formation of Malondialdehyde, 4-Hydroxynonenal, and 4-Hydroxyhexenal during in Vitro Digestion of Cooked Beef, Pork, Chicken, and Salmon. J. Agric. Food Chem. 2016, 64, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, Y.; Zou, X.; He, J.; Xu, X.; Zhou, G.; Li, C. In vitro protein digestibility of pork products is affected by the method of processing. Food Res. Int. 2017, 92, 88–94. [Google Scholar] [CrossRef]
- Narváez-Rivas, M.; Vicario, I.M.; Constante, E.G.; León-Camacho, M. Changes in the fatty acid and triacylglycerol profiles in the subcutaneous fat of Iberian ham during the dry-curing process. J. Agric. Food Chem. 2008, 56, 7131–7137. [Google Scholar] [CrossRef]
- Di Cagno, R.; Lòpez, C.C.; Tofalo, R.; Gallo, G.; De Angelis, M.; Paparella, A.; Hammes, W.P.; Gobbetti, M. Comparison of the compositional, microbiological, biochemical and volatile profile characteristics of three Italian PDO fermented sausages. Meat Sci. 2008, 79, 224–235. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Contents of polycyclic aromatic hydrocarbons (PAH) and phenolic substances in Frankfurter-type sausages depending on smoking conditions using glow smoke. Meat Sci. 2012, 90, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hitzel, A.; Pöhlmann, M.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in meat products smoked with different types of wood and smoking spices. Food Chem. 2013, 139, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Dierick, N.; Vandekerckhove, P.; Demeyer, O. Changes in Nonprotein Nitrogen Compounds during Dry Sausage Ripening. J. Food Sci. 1974, 39, 301–304. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2004, 44, 489–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German Nutrient Data Base (BLS), Version 3.01. 2010. Available online: https://www.blsdb.de/ (accessed on 31 March 2020).
- Friedman, M.; Levin, C.E.; Lee, S.-U.; Lee, J.-S.; Ohnisi-Kameyama, M.; Kozukue, N. Analysis by HPLC and LC/MS of pungent piperamides in commercial black, white, green, and red whole and ground peppercorns. J. Agric. Food Chem. 2008, 56, 3028–3036. [Google Scholar] [CrossRef]
- Li, K.; Fan, Y.; Wang, H.; Fu, Q.; Jin, Y.; Liang, X. Qualitative and quantitative analysis of an alkaloid fraction from Piper longum L. using ultra-high performance liquid chromatography-diode array detector–electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2015, 109, 28–35. [Google Scholar] [CrossRef]
- Liu, H.-L.; Luo, R.; Chen, X.-Q.; Ba, Y.-Y.; Zheng, L.; Guo, W.-W.; Wu, X. Identification and simultaneous quantification of five alkaloids in Piper longum L. by HPLC-ESI-MS(n) and UFLC-ESI-MS/MS and their application to Piper nigrum L. Food Chem. 2015, 177, 191–196. [Google Scholar] [CrossRef]
- Scott, I.M.; Puniani, E.; Jensen, H.; Livesey, J.F.; Poveda, L.; Sánchez-Vindas, P.; Durst, T.; Arnason, J.T. Analysis of Piperaceae Germplasm by HPLC and LCMS: A Method for Isolating and Identifying Unsaturated Amides from Piper spp Extracts. J. Agric. Food Chem. 2005, 53, 1907–1913. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Wedekind, R.; Keski-Rahkonen, P.; Robinot, N.; Viallon, V.; Ferrari, P.; Engel, E.; Boutron-Ruault, M.-C.; Mahamat-Saleh, Y.; Mancini, F.R.; Kühn, T.; et al. Syringol metabolites as new biomarkers for smoked meat intake. Am. J. Clin. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of Capsicum annuum (Red Sweet and Cayenne) and Piper nigrum (Black and White) Pepper Powders on the Shelf Life of Fresh Pork Sausages Packaged in Modified Atmosphere. J. Food Sci. 2006, 71, S48–S53. [Google Scholar] [CrossRef]
- Van Hecke, T.; Ho, P.L.; Goethals, S.; De Smet, S. The potential of herbs and spices to reduce lipid oxidation during heating and gastrointestinal digestion of a beef product. Food Res. Int. 2017, 102, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Macku, C.; German, J.B.; Shibamoto, T. Isolation and Identification of Dry Salami Volatiles. J. Food Sci. 1990, 55, 1239–1242. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Kim, K.; Kim, Y.-D.; Lee, S.-E. Naturally occurring Piper plant amides potential in agricultural and pharmaceutical industries: Perspectives of piperine and piperlongumine. Appl. Biol. Chem. 2019, 62, 1–7. [Google Scholar] [CrossRef]
- Playdon, M.C.; Ziegler, R.G.; Sampson, J.N.; Stolzenberg-Solomon, R.; Thompson, H.J.; Irwin, M.L.; Mayne, S.T.; Hoover, R.N.; Moore, S.C. Nutritional metabolomics and breast cancer risk in a prospective study. Am. J. Clin. Nutr. 2017, 106, 637–649. [Google Scholar] [CrossRef]
- Lécuyer, L.; Dalle, C.; Lefevre-Arbogast, S.; Micheau, P.; Lyan, B.; Rossary, A.; Demidem, A.; Petera, M.; Lagree, M.; Centeno, D.; et al. Diet-Related Metabolomic Signature of Long-Term Breast Cancer Risk Using Penalized Regression: An Exploratory Study in the SU.VI.MAX Cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 396–405. [Google Scholar] [CrossRef]
- Yengo, L.; Arredouani, A.; Marre, M.; Roussel, R.; Vaxillaire, M.; Falchi, M.; Haoudi, A.; Tichet, J.; Balkau, B.; Bonnefond, A.; et al. Impact of statistical models on the prediction of type 2 diabetes using non-targeted metabolomics profiling. Mol. Metab. 2016, 5, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Diana, M.; Quilez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic Aromatic Amines in Cooked Meat Products: Causes, Formation, Occurrence, and Risk Assessment. CRFSFS 2016, 15, 269–302. [Google Scholar] [CrossRef]
- Linseisen, J.; Rohrmann, S.; Norat, T.; Gonzalez, C.A.; Iraeta, M.D.; Gómez, P.M.; Chirlaque, M.-D.; Pozo, B.G.; Ardanaz, E.; Mattisson, I.; et al. Dietary intake of different types and characteristics of processed meat which might be associated with cancer risk – results from the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2006, 9, 449–464. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, O.; Keski-Rahkonen, P.; Chatzi, L.; Kogevinas, M.; Nawrot, T.; Pizzi, C.; Plusquin, M.; Richiardi, L.; Robinot, N.; Sunyer, J.; et al. Cord Blood Metabolic Signatures of Birth Weight: A Population-Based Study. J. Proteome Res. 2018, 17, 1235–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [Green Version]
- Haug, K.; Cochrane, K.; Nainala, V.C.; Williams, M.; Chang, J.; Jayaseelan, K.V.; O’Donovan, C. MetaboLights: A resource evolving in response to the needs of its scientific community. Nucleic Acids Res. 2020, 48, D440–D444. [Google Scholar] [CrossRef] [Green Version]




| Product | Product Type | Number of Samples | Number of Processed Products e | Number of Smoked Products | Number of Fermented Products | Number of Products from | Fat Content (g/100g Product) f | Protein Content (g/100g Product) f | |
|---|---|---|---|---|---|---|---|---|---|
| Butcher | Supermarket | ||||||||
| Lean pork a | Non-processed | 3 | 0 | 0 | 0 | 3 | 0 | 2.4 | 21.9 |
| Fatty pork b | Non-processed | 3 | 0 | 0 | 0 | 3 | 0 | 10 | 20.3 |
| Lean beef c | Non-processed | 3 | 0 | 0 | 0 | 3 | 0 | 4.2 | 21.5 |
| Fatty beef d | Non-processed | 3 | 0 | 0 | 0 | 3 | 0 | 6.6 | 22.6 |
| Cooked ham | Cured meat pieces | 3 | 3 | 0 | 0 | 1 | 2 | 3.0 ± 0.7 | 19.8 ± 1.3 |
| Raw ham | Cured meat pieces | 4 | 4 | 1 | 0 | 0 | 4 | 13.0 ± 3.5 | 28.8 ± 2.2 |
| Bacon | Cured meat pieces | 4 | 4 | 4 | 0 | 0 | 4 | 23.4 ± 3 | 16.8 ± 0.9 |
| Fried sausage | Sausage | 3 | 3 | 0 | 0 | 1 | 2 | 16.2 ± 9.3 | 15.5 ± 2.8 |
| Salami, Dry-cured sausage | Sausage | 4 | 4 | 1 | 4 | 0 | 4 | 32.8 ± 8.3 | 24.5 ± 5.1 |
| Hot dogs, frankfurter sausage | Sausage | 3 | 3 | 3 | 0 | 0 | 3 | 2.5.3± 2.5 | 12.5 ± 0.5 |
| Compound | PubChem CID | Measured m/z | RT (min) | Adjusted p-Value a | Metabolite Cluster b | Level of Confidence c | Mass Accuracy (ppm) |
|---|---|---|---|---|---|---|---|
| Piperine | 638024 | 308.1262 d | 6.01 | 3.7 × 10−5 | 4 | 1 | 2 |
| Piperyline | 636537 | 272.1287 | 5.74 | 7.4 × 10−5 | 4 | 2 | 2 |
| Piperettine | 101878852 | 312.1597 | 6.29 | 6.9 × 10−7 | 4 | 2 | 1 |
| Piperanine | 5320618 | 288.1601 | 5.93 | 8.0 × 10−8 | 4 | 2 | 2 |
| Piperdardine | 10086948 | 314.1751 | 6.28 | 1.6 × 10−7 | 4 | 2 | 0 |
| Pipercide | 5372162 | 356.222 | 6.70 | 9.3 × 10−6 | 4 | 2 | 0 |
| Pellitorine | 5318516 | 224.2012 | 6.34 | 4.8 × 10−7 | 4 | 1 | 1 |
| 4-Ethylsyringol | 61712 | 183.1018 205.0841 d | 5.08 | 0.00011 | 6 | 1 | 1 |
| 4-Methylsyringol | 240925 | 169.086 191.0686 d | 4.57 | 5.3 × 10−6 | 6 | 1 | 0 |
| 4-Allylsyringol | 5352905 | 195.102 217.0846 d | 5.32 | 0.019 | 6 | 1 | 2 |
| 4-propylsyringol | 524975 | 197.1173 219.1001 d | 5.54 | 0.020 | 6 | 1 | 0 |
| Phenylalanyl-Isoleucine | 7010566 | 279.1712 | 3.36 | 0.016 | 2 | 2 | 3 |
| Compound | m/z | RT a (min) | RT Standard (min) | Adjusted p-Value b | Mass Accuracy c (ppm) | PubChem CID | Level of Confidence d |
|---|---|---|---|---|---|---|---|
| GABA(γ-Aminobutyric acid) | 104.0709 | 0.61 | 0.59 | 0.0025 | 4 | 119 | 1 |
| Histamine | 112.0869 | 0.50 | 0.48 | 0.019 | 0 | 774 | 1 |
| Tyramine | 138.0914 | 1.31 | 1.31 | 0.016 | 2 | 5610 | 1 |
| Tryptamine | 161.1076 | 2.39 | 2.36 | 0.0025 | 2 | 1150 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wedekind, R.; Keski-Rahkonen, P.; Robinot, N.; Mercier, F.; Engel, E.; Huybrechts, I.; Scalbert, A. Metabolic Signatures of 10 Processed and Non-processed Meat Products after In Vitro Digestion. Metabolites 2020, 10, 272. https://doi.org/10.3390/metabo10070272
Wedekind R, Keski-Rahkonen P, Robinot N, Mercier F, Engel E, Huybrechts I, Scalbert A. Metabolic Signatures of 10 Processed and Non-processed Meat Products after In Vitro Digestion. Metabolites. 2020; 10(7):272. https://doi.org/10.3390/metabo10070272
Chicago/Turabian StyleWedekind, Roland, Pekka Keski-Rahkonen, Nivonirina Robinot, Frederic Mercier, Erwan Engel, Inge Huybrechts, and Augustin Scalbert. 2020. "Metabolic Signatures of 10 Processed and Non-processed Meat Products after In Vitro Digestion" Metabolites 10, no. 7: 272. https://doi.org/10.3390/metabo10070272
APA StyleWedekind, R., Keski-Rahkonen, P., Robinot, N., Mercier, F., Engel, E., Huybrechts, I., & Scalbert, A. (2020). Metabolic Signatures of 10 Processed and Non-processed Meat Products after In Vitro Digestion. Metabolites, 10(7), 272. https://doi.org/10.3390/metabo10070272