Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS Analysis of Rhodolirium Speciosum
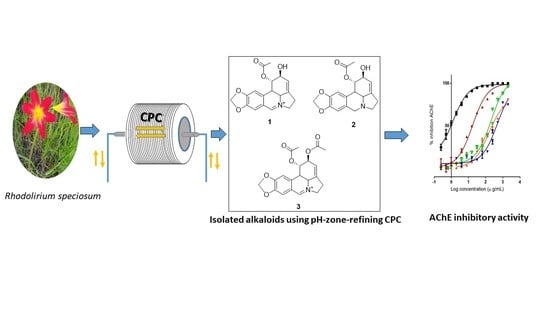
2.2. Isolation of Alkaloids by Means of pH-Zone-Refinement CPC
2.3. Isolated Alkaloids
2.4. Acethylcholinesterase Inhibition Assay
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Plant Material
3.3. Extraction and Isolation
3.4. CPC Apparatus and Separation Procedures
3.5. HPLC Analysis of CPC Fractions
3.6. Analysis of Alkaloid and CPC Fractions by GC-MS
3.7. Structural Elucidation of Isolated Alkaloids
3.8. Measurement of the Partition Coefficients (KD)
3.9. Purity Determination
3.10. Acetylcholinesterase (AChE) Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baeza, C.; Mariangel, C.; Ruiz, E.; Negritto, M. The fundamental karyotype in Rhodolirium speciosum (herb.) Ravenna and R. andicola (Poepp.) Ravenna (Amaryllidaceae). Gayana Botánica 2009, 66, 99–102. [Google Scholar] [CrossRef] [Green Version]
- Bastida, J.; Berkov, S.; Torras, L.; Pigni, N.; Andradre, J.-P.; Martínez, V.; Codina, C.; Viladomat, F. Chemical and Biological Aspects of Amaryllidaceae Alkaloids. In Recent Advances in Pharmaceutical Sciences; Transworld Research Network: Kerala, India, 2011; pp. 65–100. [Google Scholar]
- Desgagné-Penix, I. Biosynthesis of Alkaloids in Amaryllidaceae Plants: A Review. Available online: https://link.springer.com/article/10.1007/s11101-020-09678-5 (accessed on 22 July 2020).
- Moraga-Nicolás, F.; Jara, C.; Godoy, R.; Iturriaga-Vásquez, P.; Venthur, H.; Quiroz, A.; Becerra, J.; Mutis, A.; Hormazábal, E. Rhodolirium andicola: A new renewable source of alkaloids with acetylcholinesterase inhibitory activity, a study from nature to molecular docking. Rev. Bras. de Farm. 2018, 28, 34–43. [Google Scholar] [CrossRef]
- Trujillo-Chacón, L.M.; Alarcón, J.; Cespedes-Acuña, C.L.; Bustamante, L.; Baeza, M.; López, M.G.; Fernández-Mendívil, C.; Cabezas, F.; Pastene, E. Neuroprotective activity of isoquinoline alkaloids from of Chilean Amaryllidaceae plants against oxidative stress-induced cytotoxicity on human neuroblastoma SH-SY5Y cells and mouse hippocampal slice culture. Food Chem. Toxicol. 2019, 132, 110665. [Google Scholar] [CrossRef] [PubMed]
- Havelek, R.; Muthna, D.; Tomsik, P.; Kralovec, K.; Seifrtova, M.; Cahlíková, L.; Hostalkova, A.; Safratova, M.; Perwein, M.K.E.; Cermakova, E.; et al. Anticancer potential of Amaryllidaceae alkaloids evaluated by screening with a panel of human cells, real-time cellular analysis and Ehrlich tumor-bearing mice. Chem. Interact. 2017, 275, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; Rárová, L.; Strnad, M.; Bastida, J.; Cheesman, L.; Amoo, S.O. Crinane alkaloids of the amaryllidaceae with cytotoxic effects in human cervical adenocarcinoma (HeLa) cells. Nat. Prod. Commun. 2014, 9, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, J.J.; Wilhelm, A.; Bonnet, S.L.; Amoo, S.O. Antibacterial constituents of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4943–4951. [Google Scholar] [CrossRef]
- Tallini, L.R.; Bastida, J.; Cortes, N.; Osorio, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Cholinesterase Inhibition Activity, Alkaloid Profiling and Molecular Docking of Chilean Rhodophiala (Amaryllidaceae). Molecules 2018, 23, 1532. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Pigni, N.B.; Zheng, Y.; De Andrade, J.P.; Torras-Claveria, L.; Borges, W.D.S.; Viladomat, F.; Codina, C.; Bastida, J. Analysis of Bioactive Amaryllidaceae Alkaloid Profiles in Lycoris Species by GC-MS. Nat. Prod. Commun. 2014, 9, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Liu, Y.; Yang, B.; Wang, X.; Huang, L. Separation of alkaloids from herbs using high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 2545–2558. [Google Scholar] [CrossRef]
- Friesen, J.B.; Pauli, G.F. Rational development of solvent system families in counter-current chromatography. J. Chromatogr. A 2007, 1151, 51–59. [Google Scholar] [CrossRef]
- Hu, R.; Dai, X.; Lu, Y.; Pan, Y. Preparative separation of isoquinoline alkaloids from Stephania yunnanensis by pH-zone-refining counter-current chromatography. J. Chromatogr. B 2010, 878, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Toribio, A.; Delannay, E.; Richard, B.; Ple, K.; Zeches-Hanrot, M.; Nuzillard, J.M.; Renault, J.-H. Preparative isolation of huperzines A and B from Huperzia serrata by displacement centrifugal partition chromatography. J. Chromatogr. A 2007, 1140, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Geng, Y.; Li, F.; Shi, X.; Liu, J. Large-scale separation of alkaloids from Corydalis decumbens by pH-zone-refining counter-current chromatography. J. Chromatogr. A 2006, 1115, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Kukula-Koch, W.; Koch, W.; Angelis, A.; Halabalaki, M.; Aligiannis, N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of antioxidant phenolics and the isolation of alkaloids from Siberian barberry herb. Food Chem. 2016, 203, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, W.; Duan, W.; Yang, P.; Yang, B.; Jiang, J.; Li, J.; Wang, X. Preparative separation of three isoquinoline alkaloids from Berberidis radix by pH-zone-refining countercurrent chromatography. Chem. Ind. Chem. Eng. Q. 2018, 24, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Renault, J.H.; Thépenier, P.; Zèches-Hanrot, M.; Men-Olivier, L.L.; Durand, A.; Foucault, A.; Margraff, R. Preparative separation of anthocyanins by gradient elution centrifugal partition chromatograghy. J. Chromatogr. A 1997, 763, 345–352. [Google Scholar] [CrossRef]
- Ito, Y.; Ma, Y. pH-zone-refining countercurrent chromatography. J. Chromatogr. A 1996, 753, 1–36. [Google Scholar] [CrossRef]
- Weisz, A.; Mazzola, E.P.; Murphy, C.M.; Ito, Y. Preparative separation of isomeric sulfophthalic acids by conventional and pH-zone-refining counter-current chromatography. J. Chromatogr. A 2002, 966, 111–118. [Google Scholar] [CrossRef]
- Maurya, A.; Srivastava, S.K. Large-scale separation of clavine alkaloids from Ipomoea muricata by pH-zone-refining centrifugal partition chromatography. J. Chromatogr. B 2009, 877, 1732–1736. [Google Scholar] [CrossRef]
- Renault, J.-H.; Nuzillard, J.-M.; Le Crouérour, G.; Thépenier, P.; Zèches-Hanrot, M.; Le Men-Olivier, L. Isolation of indole alkaloids from Catharanthus roseus by centrifugal partition chromatography in the pH-zone refining mode. J. Chromatogr. A 1999, 849, 421–431. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, J.; Geng, Y.; Zhao, H.; Wang, D.; Wen, L. Preparative separation of quaternary ammonium alkaloids from Caulis Mahoniae by conventional and pH-zone-refining counter-current chromatography. RSC Adv. 2016, 6, 83343–83349. [Google Scholar] [CrossRef]
- Sun, C.; Duan, W.; Wang, X.; Geng, Y.; Li, J.; Wang, D. Combinative Application of pH-Zone-Refining Counter-Current Chromatography and Preparative HPLC for the Separation of Alkaloids From Lycoris radiata. J. Liq. Chromatogr. Relat. Technol. 2014, 38, 1031–1036. [Google Scholar] [CrossRef]
- Huang, S.-D.; Zhang, Y.; He, H.-P.; Li, S.-F.; Tang, G.-H.; Chen, D.-Z.; Cao, M.-M.; Di, Y.-T.; Hao, X.-J. A new Amaryllidaceae alkaloid from the bulbs of Lycoris radiata. Chin. J. Nat. Med. 2014, 11, 406–410. [Google Scholar] [CrossRef]
- Kotland, A.; Chollet, S.; Diard, C.; Autret, J.-M.; Meucci, J.; Renault, J.-H.; Marchal, L. Industrial case study on alkaloids purification by pH-zone refining centrifugal partition chromatography. J. Chromatogr. A 2016, 1474, 59–70. [Google Scholar] [CrossRef]
- Gonring-Salarini, K.; Conti, R.; De Andrade, J.P.; Borges, B.J.; Aguiar, A.C.; De Souza, J.; Zanini, C.; Oliva, G.; Tenorio, J.C.; Ellena, J.; et al. In vitro Antiplasmodial Activities of Alkaloids Isolated from Roots of Worsleya procera (Lem.) Traub (Amaryllidaceae). J. Braz. Chem. Soc. 2019, 30, 1624–1633. [Google Scholar] [CrossRef]
- Kinstle, T.H.; Wildman, W.; Brown, C. Mass spectra of amaryllidaceae alkaloids. The structure of narcissidine. Tetrahedron Lett. 1966, 7, 4659–4666. [Google Scholar] [CrossRef]
- Campbell, W.E.; Nair, J.J.; Gammon, D.W.; Bastida, J.; Codina, C.; Viladomat, F.; Smith, P.J.; Albrecht, C.F. Cytotoxic and Antimalarial Alkaloids fromBrunsvigia littoralis. Planta Medica 1998, 64, 91–93. [Google Scholar] [CrossRef]
- Tallini, L.R.; De Andrade, J.P.; Kaiser, M.; Viladomat, F.; Nair, J.J.; Zuanazzi, J.A.S.; Bastida, J. Alkaloid Constituents of the Amaryllidaceae Plant Amaryllis belladonna L. Molecules 2017, 22, 1437. [Google Scholar] [CrossRef] [Green Version]
- Feng, T.; Wang, Y.-Y.; Su, J.; Li, Y.; Cai, X.-H.; Luo, X.-D. Amaryllidaceae Alkaloids from Lycoris radiata. Helvetica Chim. Acta 2011, 94, 178–183. [Google Scholar] [CrossRef]
- Kotera, K.; Hamada, Y.; Tori, K.; Aono, K.; Kuriyama, K. Absolute configurations and the c-ring conformations of lycorine and related compounds evidenced by NMR and CD spectroscopies. Tetrahedron Lett. 1966, 7, 2009–2020. [Google Scholar] [CrossRef]
- Hao, B.; Shen, S.-F.; Zhao, Q.-J. Cytotoxic and Antimalarial Amaryllidaceae Alkaloids from the Bulbs of Lycoris radiata. Molecules 2013, 18, 2458–2468. [Google Scholar] [CrossRef] [Green Version]
- Mcnulty, J.; Nair, J.J.; Little, J.R.L.; Brennan, J.D.; Bastida, J. Structure—activity studies on acetylcholinesterase inhibition in the lycorine series of Amaryllidaceae alkaloids. Bioorg. Med. Chem. Lett. 2010, 20, 5290–5294. [Google Scholar] [CrossRef]
- Nair, J.J.; Van Staden, J. Acetylcholinesterase inhibition within the lycorine series of Amaryllidaceae alkaloids. Nat. Prod. Commun. 2012, 7, 959–962. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wan, Q.; Gu, C.; Luo, H.; Long, C. Synthesis and biological evaluation of lycorine derivatives as dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. Chem. Cent. J. 2012, 6, 96. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]





| Compound | RI | M+ | m/z (Relative Intensity %) |
|---|---|---|---|
| Lycorine Type | |||
| 11-12-dehydrolycorene | 2365 | 253(62) | 253(62), 252(100), 224(11), 166(7,5), 152(6), 139 (7) |
| Anhydrolycorine | 2508 | 251(52) | 250(100), 251(52), 220(1), 201(37), 192(14), 191(17), 165(6), 124(18) |
| 11,12-Didehydroanhydrolycorine | 2610 | 249(64) | 249(64), 248(100), 190(25), 163(9), 123(18), 96(36) |
| Sternbergine | 2716 | 331(23) | 331(23), 270(22), 252(12), 229(82), 228(100) |
| Lycorine | 2749 | 287(26) | 268(16), 250(14), 227(78), 226(100), 211(5), 147(12) |
| Dihydrolycorine | 2791 | 289(36) | 288(97), 272(28), 254(40), 214(25), 200(2), 187(22), 162(15), 147(46) |
| 2-O-Acetyllycorine | 2846 | 329(18) | 328(16), 270(42), 269(62), 268(81), 252(43), 250(100), 227(37), 226(67) |
| Acetyllycorine derivative | 2893 | 331(43) | 330(100), 270(25), 149(7) |
| Lycorine type alkaloid (2) * | 2897 | 330(<1) | 330(1), 312(1), 286(1), 270(1), 253(1), 250(1), 226(<1), 174(<1), 162 (1), 150(1), 125(100), 96(40), 83(1) |
| Lycorine type alkaloid (3) * | 2972 | 370(<1) | 280(1), 254(1), 226(1), 178(1), 147(1), 146(1),150(1), 125(100), 117(1), 108(1), 96(37), 83 (3) |
| Lycorine type alkaloid (1) * | 3045 | 327(6) | 311(1), 285(1), 270(2), 253(3), 226(2), 150(1) 125(100), 108(1), 96(40), 83(7) |
| Haemanthamine Type | |||
| Vittatine | 2471 | 271(82) | 272(14), 252(16), 199(89), 187(40), 173(14), 115(22) |
| 8-O-Demethylmaritidine | 2517 | 273(100) | 230(5), 202(29), 201(74), 189(10), 175(12), 174(31), 129(13), 128(21), 115(46), 56(13) |
| Aulicine | 2616 | 304(23) | 304(23), 288(47), 233(96), 206(60), 190(30), 175(11), 163(23) |
| Haemanthamine | 2634 | 301(23) | 272(100), 257(12), 240(26), 225(16), 211(24), 181(44), 153(13) |
| Galanthamine Type | |||
| Galanthamine | 2406 | 287(77) | 287 (41) 286 (47), 244 (10),230 (10), 216 (22), 174 (23), 128 (11), 115 (12) |
| Norlycoramine | 2460 | 275(57) | 274(95), 273(51), 202(53), 188(13), 173(20), 160(29), 115(20) |
| Not identified | |||
| Nerinine type alkaloid (1) | 2484 | 281(1) | 271(3), 254(2), 238(1), 207(1), 128(2), 115(2), 109(100), 108(14) |
| Homolycorine type alkaloid (6) | 2401 | 281(8) | 281 (8) 250 (5), 222(4), 147 (23), 129 (100), 112(25), 83 (18), 70 (33), 57(39) |
| Galantathamine derivate 1 | 2547 | 289(97) | 289(97), 272(15), 244(10), 230(14), 218(52), 216(17), 174(11), 149(14), 128 (29), 115 (28) |
| Galanthamine derivate 2 | 2601 | 344(100) | 345(38), 344(100), 251(2), 248(2), 226(8), 161(7), 147(3), 129(5), 101(6) |
| Galanthamine derivate 3 | 2654 | 306(22) | 306(22), 305(100), 290(16), 288(22), 276(27), 248(21), 233(72), 206(47), 175(14) |
| # | Solvent Systems a | Retentor (TEA) b | Displacer (Acid) |
|---|---|---|---|
| 1 | n-Hept/EtOAc/n-PrOH/W | 15 mM | HCl 6 mM |
| 2 | n-Hept/EtOAc/n-PrOH/W | 15 mM | Acetic acid 6 mM |
| 3 | n-Hept/EtOAc/n-PrOH/W | 15 mM | Formic acid 3 mM |
| 4 | MtBE/ACN/W | 12 mM | Formic acid 6 mM |
| 5 | MtBE/ACN/W | 15 mM | Formic acid 3 mM |
| Alkaloid 1 | Alkaloid 2 | |
|---|---|---|
| KD | 0.64 | 0.40 |
| Kbasic (15 mM TEA) | 0.50 | 0.20 |
| Kacid (3 mM formic acid) | 24.42 | 4.38 |
| Kacid (6 mM Acetic acid) | 30.79 | 7.39 |
| Alkaloid 1 | Alkaloid 2 | Alkaloid 3 | |
|---|---|---|---|
| KD | 2.99 | 12.24 | 1.42 |
| Kacid | 33.76 | 48.65 | 12.52 |
| Kbase | 1.35 | 0.74 | 0.43 |
| Dry weight (mg) | 65.7 | 50.1 | 12.3 |
| Purity (%) | 88.2 | 97.7 | 84.4 |
| Yield (%) | 1.8 | 9.0 | 2.2 |
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| Position | δH | δC | δH | δC | δ | δ |
| 1 | 4.66 bs | 66.41 | 5.93 bs | 67.50 | 4.62 s | 66.8 |
| 2 | 4.40 bs | 80.48 | 4.15 bs | 81.56 | 4.38 s | 80.34 |
| 3 | 5.83 s | 119.28 | 5.58 s | 120.05 | 5.77 s | 120.1 |
| 4 | - | 152.68 | - | 141.98 | - | 151.7 |
| 4a | 2.90 d (10) | 60.62 | 2.75 d (10) | 66.42 | 3.90 d (10) | 60.7 |
| 6α | 8.47 s | 168.15 | 4.23 d (13) | 56.4 | 8.55 s | 164.7 |
| 6β | - | 3.70 d (14) | - | - | ||
| 6a | - | 139.73 | - | 138.4 | - | 139.2 |
| 7 | 7.07 | 109.64 | 6.96 s | 110.08 | 7.01 | 109.8 |
| 8 | - | 145.55 | - | 152.05 | - | 146.2 |
| 9 | - | 143.48 | - | 148.20 | - | 143.1 |
| 10 | 7.51 | 103.45 | 7.50 s | 108.74 | 7.49 s | 102.9 |
| 10a | - | 136.93 | - | 118.5 | - | 136.5 |
| 10b | 2.99 bd (10.7) | 43.00 | 2.87 d (10) | 42.79 | 2.99 | 42.8 |
| 11 α,β | 2.66 m | 27.60 | 2.77 s | 27.46 | 2.85 | 27.6 |
| 12 α | 2.53 dd (15.1, 9.0) | 55.77 | 3.24 m | 55.59 | 3.75 | 56.1 |
| 12 β | 3.49 m | - | - | |||
| O-CH2-O | 6.11 d (1.2) | 101.9 | 6.10 d (1.1) | 102.26 | 6.05 bs | 102.2 |
| O-CO-CH3 | 2.30 s | 39.66 | 2.07 s | 37.55 | 2.67 | 20.9 |
| 161.33 | 164.38 | 169.7 | ||||
| O-CO-CH3 | 2.11 | 21.1 | ||||
| 170.0 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa, D.I.; Pastene-Navarrete, E.; Bustamante, L.; Baeza, M.; Alarcón-Enos, J. Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities. Metabolites 2020, 10, 309. https://doi.org/10.3390/metabo10080309
Correa DI, Pastene-Navarrete E, Bustamante L, Baeza M, Alarcón-Enos J. Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities. Metabolites. 2020; 10(8):309. https://doi.org/10.3390/metabo10080309
Chicago/Turabian StyleCorrea, Diana Isabel, Edgar Pastene-Navarrete, Luis Bustamante, Marcelo Baeza, and Julio Alarcón-Enos. 2020. "Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities" Metabolites 10, no. 8: 309. https://doi.org/10.3390/metabo10080309
APA StyleCorrea, D. I., Pastene-Navarrete, E., Bustamante, L., Baeza, M., & Alarcón-Enos, J. (2020). Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities. Metabolites, 10(8), 309. https://doi.org/10.3390/metabo10080309