Effects of Water Deficit and Heat Stress on Nicotiana langsdorffii Metabolomic Pattern Modified by Insertion of rolD Gene from Agrobacterium rhizogenes
Abstract
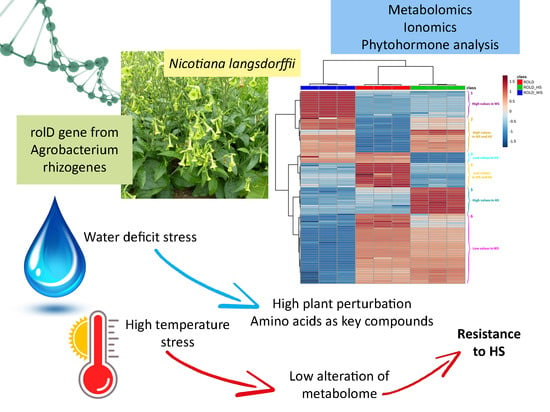
:1. Introduction
2. Results and Discussion
2.1. Dry Weight and Chemical Characterization
2.2. Determination of Elements
2.3. Metabolites That Vary in Both WS and HS (Clusters 2 and 4)
2.4. Metabolites That Vary Only in WS (Clusters 1 and 6)
2.5. Metabolites That Vary Only in HS (Clusters 3 and 5)
3. Materials and Methods
3.1. Sample Preparation
3.1.1. Plant Growth and Genetic Modification
3.1.2. Stress Inductions
3.1.3. Plant Material Preparation
3.2. Metabolomics Analysis
3.2.1. Experimental Procedure and Quality Control
3.2.2. Data Treatment and Metabolite Identification
3.3. Phytohormone Analysis
3.4. Elemental Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme Temperature Responses, Oxidative Stress and Antioxidant Defense in Plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; 2009; pp. 169–205. ISBN 978-953-51-1024-8. [Google Scholar]
- Larkindale, J.; Huang, B. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2004, 51, 57–67. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [Green Version]
- Arbona, V.; Manzi, M.; De Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophysics 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Malandrino, M.; Giacomino, A.; Karthik, M.; Zelano, I.; Fabbri, D.; Ginepro, M.; Fuoco, R.; Bogani, P.; Abollino, O. Inorganic markers profiling in wild type and genetically modified plants subjected to abiotic stresses. Microchem. J. 2017, 134, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Giannarelli, S.; Muscatello, B.; Bogani, P.; Spiriti, M.M.; Buiatti, M.; Fuoco, R. Comparative determination of some phytohormones in wild-type and genetically modified plants by gas chromatography-mass spectrometry and high-performance liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2010, 398, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Irdani, T.; Caroppo, S.; Ambrogioni, L. Response of Nicotiana tabacum plants overexpressing a glucocorticoid receptor to Meloidogyne incognita (Nematoda Tylenchida) infestation. Redia 2003, 86, 35–38. [Google Scholar]
- Scalabrin, E.; Radaelli, M.; Capodaglio, G. Simultaneous determination of shikimic acid, salicylic acid and jasmonic acid in wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses. Plant Physiol. Biochem. 2016, 103, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Scalabrin, E.; Radaelli, M.; Rizzato, G.; Bogani, P.; Buiatti, M.; Gambaro, A.; Capodaglio, G. Metabolomic analysis of wild and transgenic Nicotiana langsdorffii plants exposed to abiotic stresses: Unraveling metabolic responses. Anal. Bioanal. Chem. 2015, 407, 6357–6368. [Google Scholar] [CrossRef]
- Bettini, P.; Michelotti, S.; Bindi, D.; Giannini, R.; Capuana, M.; Buiatti, M. Pleiotropic effect of the insertion of the Agrobacterium rhizogenes rolD gene in tomato (Lycopersicon esculentum Mill.). Theor. Appl. Genet. 2003, 107, 831–836. [Google Scholar] [CrossRef]
- Palazón, J.; Cusidó, R.M.; Roig, C.; Piñol, M.T. Expression of the rol C gene and nicotine production in transgenic roots and their regenerated plants. Plant Cell Rep. 1998, 17, 384–390. [Google Scholar] [CrossRef]
- Del Bubba, M.; Ancillotti, C.; Checchini, L.; Ciofi, L.; Fibbi, D.; Gonnelli, C.; Mosti, S. Chromium accumulation and changes in plant growth, selected phenolics and sugars of wild type and genetically modified Nicotiana langsdorffii. J. Hazard. Mater. 2013, 262, 394–403. [Google Scholar] [CrossRef]
- Mauro, M.L.; Costantino, P.; Bettini, P.P. The never ending story of rol genes: A century after. Plant Cell. Tissue Organ Cult. 2017, 131, 201–212. [Google Scholar] [CrossRef]
- Fuoco, R.; Bogani, P.; Capodaglio, G.; Del Bubba, M.; Abollino, O.; Giannarelli, S.; Spiriti, M.M.; Muscatello, B.; Doumett, S.; Turetta, C.; et al. Response to metal stress of Nicotiana langsdorffii plants wild-type and transgenic for the rat glucocorticoid receptor gene. J. Plant Physiol. 2013, 170, 668–675. [Google Scholar] [CrossRef]
- Ardini, F.; Soggia, F.; Abelmoschi, M.L.; Magi, E.; Grotti, M. Effect of heat stress on the ionomic profile of Nicotiana langsdorffii wild-type and mutant genotypes. Int. J. Environ. Anal. Chem. 2016, 96, 460–473. [Google Scholar] [CrossRef]
- Ancillotti, C.; Bogani, P.; Biricolti, S.; Calistri, E.; Checchini, L.; Ciofi, L.; Gonnelli, C.; Del Bubba, M. Changes in polyphenol and sugar concentrations in wild type and genetically modified Nicotiana langsdorffii Weinmann in response to water and heat stress. Plant Physiol. Biochem. 2015, 97, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ranaldo, M.; Toscano, G.; Radaelli, M.; Scalabrin, E.; Capodaglio, G. Nicotiana langsdorffii wild type and genetically modified exposed to chemical and physical stress: Changes in element content. Int. J. Environ. Anal. Chem. 2015, 95, 349–365. [Google Scholar] [CrossRef] [Green Version]
- Trovato, M.; Maras, B.; Linhares, F.; Costantino, P. The plant oncogene rolD encodes a functional ornithine cyclodeaminase. Proc. Natl. Acad. Sci. USA 2001, 98, 13449–13453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaletti, A.; Naghavi, M.R.; Toorchi, M.; Zolla, L.; Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 2018, 8, 5710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Du, X.; Ren, Y.; Wylie, S.J. Metabolic responses of endophytic Nicotiana benthamiana plants experiencing water stress. Environ. Exp. Bot. 2017, 143, 59–71. [Google Scholar] [CrossRef]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D. Salicylic acid signaling in disease resistance. Plant Sci. 2014, 228, 127–134. [Google Scholar] [CrossRef]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E.; Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat Stress Phenotypes of Arabidopsis Mutants Implicate Multiple Signaling Pathways in the Acquisition of Thermotolerance Published by: American Society of Plant Biologists (ASPB) Stable URL: https://www.jstor.org/stable/4629891 REFERENCES Linked refe. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.J.M.; Wasternack, C.; Mur, L.A. Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Murakami, Y. Trienoic Fatty Acids and Plant Tolerance of High Temperature. Science 2000, 287, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, M.; Yang, R.; Luo, Q.; Xu, J.; Ye, Y.; Yan, X. Profiling lipidome changes of Pyropia haitanensis in short-term response to high-temperature stress. J. Appl. Phycol. 2016, 28, 1903–1913. [Google Scholar] [CrossRef]
- Cowan, A.K. Phospholipids as plant growth regulators. Plant Growth Regul. 2006, 48, 97–109. [Google Scholar] [CrossRef]
- Ryu, S.B.; Karlsson, B.H.; Ozgen, M.; Palta, J.P. Inhibition of phospholipase D by lysophosphatidylethanolamine, a lipid-derived senescence retardant. Proc. Natl. Acad. Sci. USA 1997, 94, 12717–12721. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Bi, A.; Hu, Z.; Amombo, E.; Li, H.; Fu, J. Antioxidant metabolism, photosystem ii, and fatty acid composition of two tall fescue genotypes with different heat tolerance under high temperature stress. Front. Plant Sci. 2018, 9, 1242. [Google Scholar] [CrossRef] [Green Version]
- Tarazona, P.; Feussner, K.; Feussner, I. An enhanced plant lipidomics method based on multiplexed liquid chromatography-mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling. Plant J. 2015, 84, 621–633. [Google Scholar] [CrossRef]
- Joshi, V.; Joung, J.G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardhana, P.; Ren, L.; Amarasinghe, V.; Monaco, M.; Thomason, J.; Ravenscroft, D.; McCouch, S.; Ware, D.; Jaiswal, P. A genome scale metabolic network for rice and accompanying analysis of tryptophan, auxin and serotonin biosynthesis regulation under biotic stress. Rice 2013, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielach, A.; Hrtyan, M.; Tognetti, V.B. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yang, A.; Yin, H.; Zhang, J. Influence of Water Stress on Endogenous Hormone Contents and Cell Damage of Maize Seedlings. J. Integr. Plant Biol. 2008, 50, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Rushton, P.; Rohila, J. Metabolomic Profiling of Soybeans (Glycine max L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, G.A.; Zaplachinski, S.T. The effects of drought stress on free amino acid accumulation and protein synthesis in Brassica napus. Physiol. Plant. 1994, 90, 9–14. [Google Scholar] [CrossRef]
- Moradi, P.; Ford-Lloyd, B.; Pritchard, J. Metabolomic approach reveals the biochemical mechanisms underlying drought stress tolerance in thyme. Anal. Biochem. 2017, 527, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef]
- Vincent, D.; Lapierre, C.; Pollet, B.; Cornic, G.; Negroni, L.; Zivy, M. Water Deficits Affect Caffeate O-Methyltransferase, Lignification, and Related Enzymes in Maize Leaves. A Proteomic Investigation. Plant Physiol. 2005, 137, 949–960. [Google Scholar] [CrossRef] [Green Version]
- Bassard, J.E.; Ullmann, P.; Bernier, F.; Werck-Reichhart, D. Phenolamides: Bridging polyamines to the phenolic metabolism. Phytochemistry 2010, 71, 1808–1824. [Google Scholar] [CrossRef]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Macoy, D.M.; Kim, W.Y.; Lee, S.Y.; Kim, M.G. Biosynthesis, physiology, and functions of hydroxycinnamic acid amides in plants. Plant Biotechnol. Rep. 2015, 9, 269–278. [Google Scholar] [CrossRef]
- Kim, J.; Kang, K.; Gonzales-vigil, E.; Shi, F.; Jones, A.D.; Barry, C.S.; Last, R.L. Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the acyltransferase2 Locus in the wild tomato Solanum habrochaites. Plant Physiol. 2012, 160, 1854–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glas, J.J.; Schimmel, B.C.J.; Alba, J.M.; Escobar-bravo, R.; Schuurink, R.C.; Kant, M. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.X.; Fu, W.W.; Li, W.; Bi, K.S.; Wang, M.W. Diosgenin glucuronides from Solanum lyratum and their cytotoxicity against tumor cell lines. Z. Naturforsch. Sect. C J. Biosci. 2006, 61, 171–176. [Google Scholar] [CrossRef]
- Kumar, P.M.; Murugan, K.; Kovendan, K.; Subramaniam, J.; Amaresan, D. Mosquito larvicidal and pupicidal efficacy of Solanum xanthocarpum (Family: Solanaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2541–2550. [Google Scholar] [CrossRef]
- El-Sayed, A.A.; Razin, A.M.; Swaefy, H.M.F.; Mohamed, S.M.; Abou-Aitah, K.E.A. Effect of Water Stress on Yield and Bioactive Chemical Constituents of Tribulus Species. J. Appl. Sci. Res. 2008, 4, 2134–2144. [Google Scholar]
- Narula, A.; Kumar, S.; Srivastava, P.S. Abiotic metal stress enhances diosgenin yield in Dioscorea bulbifera L. cultures. Plant Cell Rep. 2005, 24, 250–254. [Google Scholar] [CrossRef]
- Parent, G.J.; Giguère, I.; Mageroy, M.; Bohlmann, J.; MacKay, J.J. Evolution of the biosynthesis of two hydroxyacetophenones in plants. Plant Cell Environ. 2018, 41, 620–629. [Google Scholar] [CrossRef]
- Miguel, M.G.; Barroso, J.G. Accumulation of stress metabolites in cell suspension cultures of Hyoscyamus albus. Phytochemistry 1994, 35, 371–375. [Google Scholar] [CrossRef]
- Chong, J.; Pierrel, M.-A.; Atanassova, R.; Werck-Reichart, D.; Fritig, B.; Saindrenan, P. Free and Conjugated Benzoic Acid in Tobacco Plants and Cell Cultures. Induced Accumulation upon Elicitation of Defense Responses and Role as Salicylic Acid Precursors. Plant Physiol. 2001, 125, 318–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Väisänen, E.E.; Smeds, A.I.; Fagerstedt, K.V.; Teeri, T.H.; Willför, S.M.; Kärkönen, A. Coniferyl alcohol hinders the growth of tobacco BY-2 cells and Nicotiana benthamiana seedlings. Planta 2015, 242, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, A.V.; Bloem, E.; Brown, P.H.; Bryson, G.M.; Datnoff, L.E.; De Kok, L.J.; Drihem, K.; Dunn, M.A.; Gorham, J.; Graham, R.D.; et al. Handbook of Plant Nutrition; Barker, A.V., Pilbeam, J.D., Eds.; Taylor and Francis CRC: Boca Raton, FL, USA, 2007; ISBN 0-8247-5904-4. [Google Scholar]
- Meisch, H.; Becker, L.J.M. Vanadium in photosynthesis of Chlorella fusca and higher plants. Biochim. Biophys. Acta 1981, 636, 119–125. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Martinec, J.; Ruelland, E. The phosphatidic acid paradox: Too many actions for one molecule class? Lessons from plants. Prog. Lipid Res. 2018, 71, 43–53. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 2019, 439, 57–73. [Google Scholar] [CrossRef] [Green Version]
- Browse, J.; Warwick, N.; Somerville, C.R.; Slack, C.R. Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3′ plant Arabidopsis thaliana. Biochem. J. 1986, 235, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef]
- Simon-Sarkadi, L.; Kocsy, G.; Várhegyi, Á.; Galiba, G.; De Ronde, J.A. Stress-induced changes in the free amino acid composition in transgenic soybean plants having increased proline content. Biol. Plant. 2006, 50, 793–796. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis Enzyme Family That Conjugates Amino Acids to Indole-3-Acetic Acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianfagna, T.J.; Davies, P.J. The transport of substances out of developing fruits in relation to the induction of apical senescence in Pisum sativum line G2. Physiol. Plant. 1983, 59, 676–684. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torras-Claveria, L.; Jáuregui, O.; Codina, C.; Tiburcio, A.F.; Bastida, J.; Viladomat, F. Analysis of phenolic compounds by high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry in senescent and water-stressed tobacco. Plant Sci. 2012, 182, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Watanabe, T.; Shinano, T.; Ezawa, T.; Wasaki, J.; Kimura, K.; Osaki, M.; Zhu, Y.G. Element interconnections in Lotus japonicus: A systematic study of the effects of element additions on different natural variants. Soil Sci. Plant Nutr. 2009, 55, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef]
- Kant, S.; Kafkafi, U. Potassium and Abiotic Stresses in Plants. In Potassium for Sustainable Crop Production; Potash Inst.: Gurgaon, India, 2002; pp. 233–251. [Google Scholar]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. Water Stress Crop Plants A Sustain. Approach 2016, 2, 649–668. [Google Scholar] [CrossRef]
- Sun, G.; Strebl, M.; Merz, M.; Blamberg, R.; Huang, F.C.; McGraphery, K.; Hoffmann, T.; Schwab, W. Glucosylation of the phytoalexin N-feruloyl tyramine modulates the levels of pathogen-responsive metabolites in Nicotiana benthamiana. Plant J. 2019, 100, 20–37. [Google Scholar] [CrossRef]
- Han, F.; Chen, H.; Li, X.J.; Yang, M.F.; Liu, G.S.; Shen, S.H. A comparative proteomic analysis of rice seedlings under various high-temperature stresses. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 1625–1634. [Google Scholar] [CrossRef]
- Marti, G.; Erb, M.; Boccard, J.; Glauser, G.; Doyen, G.R.; Villard, N.; Robert, C.A.M.; Turlings, T.C.J.; Rudaz, S.; Wolfender, J.L. Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant. Cell Environ. 2013, 36, 621–639. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.V.; Welti, R. Wheat leaf lipids during heat stress: II. Lipids experiencing coordinated metabolism are detected by analysis of lipid co-occurrence. Plant Cell Environ. 2016, 39, 608–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuru, M.; Yu, Y.; Mizoi, J.; Kawamoto-fujioka, M.; Wang, J.; Fujiki, Y.; Nishida, I. Mitochondrial Phosphatidylethanolamine Level Modulates Cyt c Oxidase Activity to Maintain Respiration Capacity in Arabidopsis thaliana Rosette Leaves. Plant Cell Physiol. 2013, 54, 1612–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeem, M.; Khan, A.; Masroor, M. Moinuddin Triacontanol: A potent plant growth regulator in agriculture. J. Plant Interact. 2012, 7, 129–142. [Google Scholar] [CrossRef]
- Waqas, M.; Shahzad, R.; Khan, A.L.; Asaf, S.; Kim, Y.H.; Kang, S.M.; Bilal, S.; Hamayun, M.; Lee, I.J. Salvaging effect of triacontanol on plant growth, thermotolerance, macro-nutrient content, amino acid concentration and modulation of defense hormonal levels under heat stress. Plant Physiol. Biochem. 2016, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiol. Plant. 2014, 36, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Horsch, R.B.; Fry, J.; Hoffmann, N.; Neidermeyer, J.; Rogers, S.G.; Fraley, R.T. Leaf disc transformation. Plant Mol. Biol. Man. 1988, 9, 79–87. [Google Scholar] [CrossRef]
- Lommen, A. MetAlign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef]
- Lommen, A.; Van der Kamp, H.J.; Kools, H.J.; Van der Lee, M.K.; Van der Weg, G.; Mol, H.G.J. metAlignID: A high-throughput software tool set for automated detection of trace level contaminants in comprehensive LECO two-dimensional gas chromatography time-of-flight mass spectrometry data. J. Chromatogr. A 2012, 1263, 169–178. [Google Scholar] [CrossRef]
- Tikunov, Y.M.; Laptenok, S.; Hall, R.D.; Bovy, A.; De Vos, R.C.H. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 2012, 8, 714–718. [Google Scholar] [CrossRef] [Green Version]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| FW | DW | |
|---|---|---|
| RolD | 3.3 | 0.216 * |
| SD | 1.2 | 0.08 |
| RolDWS | 3.4 | 0.34 |
| SD | 2.4 | 0.2 |
| RolDHS | 4.21 | 0.378 * |
| SD | 0.6 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalabrin, E.; Radaelli, M.; Capodaglio, G. Effects of Water Deficit and Heat Stress on Nicotiana langsdorffii Metabolomic Pattern Modified by Insertion of rolD Gene from Agrobacterium rhizogenes. Metabolites 2020, 10, 310. https://doi.org/10.3390/metabo10080310
Scalabrin E, Radaelli M, Capodaglio G. Effects of Water Deficit and Heat Stress on Nicotiana langsdorffii Metabolomic Pattern Modified by Insertion of rolD Gene from Agrobacterium rhizogenes. Metabolites. 2020; 10(8):310. https://doi.org/10.3390/metabo10080310
Chicago/Turabian StyleScalabrin, Elisa, Marta Radaelli, and Gabriele Capodaglio. 2020. "Effects of Water Deficit and Heat Stress on Nicotiana langsdorffii Metabolomic Pattern Modified by Insertion of rolD Gene from Agrobacterium rhizogenes" Metabolites 10, no. 8: 310. https://doi.org/10.3390/metabo10080310
APA StyleScalabrin, E., Radaelli, M., & Capodaglio, G. (2020). Effects of Water Deficit and Heat Stress on Nicotiana langsdorffii Metabolomic Pattern Modified by Insertion of rolD Gene from Agrobacterium rhizogenes. Metabolites, 10(8), 310. https://doi.org/10.3390/metabo10080310