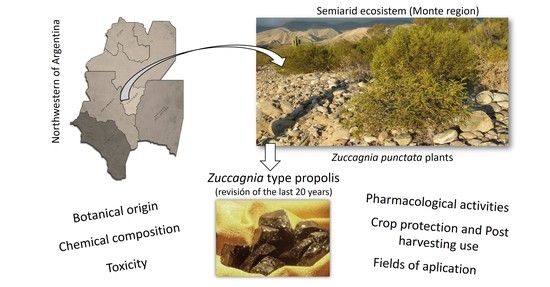
Propolis from the Monte Region in Argentina: A Potential Phytotherapic and Food Functional Ingredient
Abstract
:1. Introduction
2. Research on Argentine Propolis
2.1. Propolis from the Monte Region in Argentina
2.1.1. Chemical Characterization of Propolis from the Monte Region
2.1.2. Botanical Origin by Microscopic Analyses and Chemical Analysis
2.1.3. Pharmacological Activities
2.1.3.1. Antibacterial Activity
2.1.3.2. Antifungal Effect
2.1.3.3. Nematicidal Activities
2.1.3.4. Antioxidant Capacity
2.1.3.5. Effects on Pro-Inflammatory Mediators
2.1.3.6. Inhibitory Capacity of Enzymes Related to Metabolic Syndrome
2.1.4. Crop Protection and Post Harvesting Use
Antibacterial Activity
Antifungal Activity
2.1.5. Toxicity of Propolis from the Monte Region
2.1.6. Fields of Application of Propolis from the Monte Region
3. Concluding Remarks and Future Trends
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bankova, V.; Popova, M.; Trusheva, B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis—chemical-composition, biological properties and therapeutic activity. Apidologie 1995, 2, 83–99. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–636. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Recent trends and important developments in propolis research. eCAM 2005, 2, 29–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sforcin, J.M. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. JAAS 2009, 1, 23–28. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cen. J. 2014, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Almuhayawi, M.S. Propolis: A Novel Antibacterial Agent. Saudi J. Biol. Sci. 2020, 27, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Ranjbar, T.; Mosallanezhad, Z.; Mahmoodi, M.; Moosavian, S.P.; Ferns, G.; Jalali, Z.; Sohrabi, Z. Effect of Propolis supplementation on serum CRP and TNF-α levels in adults: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2020, 50, 102380. [Google Scholar] [CrossRef] [PubMed]
- Rojczyk, E.; Klama-Baryła, A.; Łabuś, W.; Wilemska-Kucharzewska, K.; Kucharzewski, M. Historical and modern research on propolis and its application in wound healing and other fields of medicine and contributions by Polish studies. J. Ethnopharmacol. 2020, 262, 113159. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Chapter Two—Propolis flavonoids and terpenes, and their interactions with model lipid membranes: A review. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2020; Volume 32, pp. 25–52. [Google Scholar]
- Stavropoulou, I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR metabolic profiling of Greek propolis samples: Comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharm. Biomed. 2021, 194, 113814. [Google Scholar] [CrossRef]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Filho, G.C.L.; Bueno-Silva, B.; de Moraes, J. Brazilian red propolis exhibits antiparasitic properties in vitro and reduces worm burden and egg production in a mouse model harboring either early or chronic Schistosoma mansoni infection. J. Ethnopharmacol. 2020, 264, 113387. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced Type 2 Diabetes in rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Berretta, A.A.; Duarte Silveira, M.A.; Cóndor Capcha, J.M.; De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharm. 2020, 131, 110622. [Google Scholar] [CrossRef]
- Rosseto, H.C.; de Toledo, L.D.A.S.; dos Santos, R.S.; de Francisco, L.M.B.; Vecchi, C.F.; Esposito, E.; Cortesi, R.; Bruschi, M.L. Design of propolis-loaded film forming systems for topical administration: The effect of acrylic acid derivative polymers. J. Mol. Liq. 2020, 114514. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Martïn-Alvarez, P.J.; Señoráns, F.J.; Reglero, G.; Capodicasa, A.; Nazzaro, F.; Sada, A.; Cifuentes, A.; Elena Ibáñez, E. Design of natural food antioxidant ingredients through a chemometric approach. J. Agric. Food Chem. 2010, 58, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, F.C.; da Fonseca, C.R.; de Alencar, S.M.; Thomazini, M.; Balieiro, J.C.D.D.; Pittia, P.; Favaro-Trindade, C.S. Assessment of production efficiency, physicochemical properties and storage stability of spray-dried propolis, a natural food additive, using gum Arabic and OSA starch-based carrier systems. Food Bioprod. Process. 2013, 91, 28–36. [Google Scholar] [CrossRef]
- Yücel, B.; Topal, E.; Kosoglu, M. Bee Products as Functional Food. In Superfood and Functional Food—An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017; pp. 16–33. [Google Scholar]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Sadhana, N.; Lohidasan, S.; Mahadik, K. Marker-based standardization and investigation of nutraceutical potential of Indian propolis. J. Integr. Med. 2017, 15, 483–494. [Google Scholar] [CrossRef]
- Argentine Food Code. 2009 Cap. XVII. Art. 1384. Available online: http://www.anmat.gov.ar/alimentos/codigoa/Capitulo_XVII.pdf (accessed on 12 December 2020).
- Bankova, V.; De Castro, L.; Marcucci, M.C. Propolis recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Review Advances in the Propolis chemical composition between 2013 and 2018: A Review. eFood 2020, 1, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Salatino, A.; Teixeira, E.W.; Negri, G.; Message, D. Origin and chemical variation of Brazilian propolis. Evid. Based Complementary Altern. Med. 2005, 2, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenaway, W.; Scaysbrook, T.; Whately, F.R. The composition and plant origin of propolis: A report of work at Oxford. Bee World 1990, 71, 107–118. [Google Scholar] [CrossRef]
- Lotti, C.; Campo Fernández, M.; Piccinelli, A.L.; Cuesta-Rubio, O.; Márquez Hernández, I.; Rastrelli, L. Chemical constituents of red Mexican propolis. J. Agric. Food Chem. 2010, 58, 2209–2213. [Google Scholar] [CrossRef]
- Tomas-Barberán, F.A.; Garcia-Viguera, C.; Vitolivier, P.; Ferreres, F.; Tomás-Lorente, F. Phytochemical evidence for the botanical origin of tropical propolis from Venezuela. Phytochemistry 1993, 34, 191–196. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Piccinelli, A.L.; Campo Fernandez, M.; Marquez Hernandez, I.; Rosado, A.; Rastrelli, L. Chemical characterization of Cuban propolis by HPLC-PDA, HPLC-MS, and NMR: The brown, red, and yellow cuban varieties of propolis. J. Agric. Food Chem. 2007, 55, 7502–7509. [Google Scholar] [CrossRef]
- Koenig, B. Plant sources of propolis. Bee World 1995, 66, 136–139. [Google Scholar] [CrossRef]
- Montenegro, G.; Peña, R.C.; Mujica, A.M.; Pizarro, R. Botanical resources for propolis in an apiary network in central Chile. Phyton Int. J. Exp. Bot. 2001, 70, 191–201. [Google Scholar]
- Isla, M.I.; Nieva Moreno, M.I.; Zampini, I.C.; Solórzano, E.; Danert, F.; Vera, N.; Sayago, J.E.; Bedascarrabure, E.; Maldonado, L.; Ordoñez, R. Argentine propolis: Its flavonoid and chalcone content and its relation with the functional properties. In Beneficial Effects of Propolis on Human Health and Chronic Diseases; Farooqui, T., Farooqui, A., Eds.; Nova Science Publisher: Hauppauge, NY, USA, 2011; Volume 8, pp. 161–169. [Google Scholar]
- Isla, M.I.; Paredes Guzman, J.F.; Nieva Moreno, M.I.; Koo, H.; Park, Y.K. Some chemical composition and biological activity of Northern Argentine propolis. Some chemical composition and biological activity of Northern Argentine propolis. J. Agric. Food Chem. 2005, 53, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Nieva Moreno, M.I.; Zampini, I.C.; Ordóñez, M.; Vattuone, M.A.; Isla, M.I. Evaluation of the cytotoxicity, mutagenicity and antimutagenicity of propolis from Amaicha del Valle, Tucumán, Argentina. J. Agric. Food Chem. 2005, 53, 8957–8962. [Google Scholar] [CrossRef]
- Chaillou, L.; Nazareno, M. Bioactivity of propolis from Santiago del Estero, Argentina, related to their chemical composition LWT. Food Sci. Tech. 2009, 42, 1422–1427. [Google Scholar]
- Agüero, M.B.; González, M.; Lima, B.; Svetaz, L.; Sánchez, M.; Zacchino, S.; Feresin, G.; Schmeda-Hirschmann, G.; Palermo, J.; Wunderlin, D.; et al. Argentinean propolis from Zuccagnia punctata Cav. (Caesalpinieae) exudates: Phytochemical characterization and antifungal activity. J. Agric. Food Chem. 2010, 58, 194–201. [Google Scholar]
- Vera, N.; Solórzano, E.; Ordóñez, R.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Chemical composition of Argentinean propolis collected in extreme regions and its relation with antimicrobial and antioxidant activities. Nat. Prod. Commun. 2011, 6, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Danert, F.C.; Zampini, C.; Ordoñez, R.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Argentinean Propolis as non conventional functional foods. Nutritional and functional composition. Nat. Prod. Commun. 2014, 9, 167–170. [Google Scholar]
- Salas, A.L.; Ordóñez, R.M.; Silva, C.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I.; Zampini, I.C. Antimicrobial activity of Argentinean propolis against Staphylococcus isolated of canine otitis. J. Exp. Biol. Agric. Sci. 2014, 2, 197–207. [Google Scholar]
- Salas, A.L.; Alberto, M.R.; Zampini, I.C.; Cuello, A.S.; Maldonado, L.; Ríos, J.L.; Isla, M.I. Biological activities of polyphenols-enriched propolis from Argentina arid regions. Phytomedicine 2016, 23, 27–31. [Google Scholar] [CrossRef]
- Solórzano, E.; Vera, N.; Cuello, S.; Ordóñez, R.; Zampini, C.; Maldonado, L.; Bedascarrasbure, E.; Isla, M.I. Chalcones in bioactive Argentine propolis collected in arid environments. Nat. Prod. Commun. 2012, 7, 879–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solórzano, E.R.; Bortolini, C.; Bogialli, S.; Di Gangi, I.M.; Favaro, G.; Maldonado, L.; Pastore, P. Use of a LC-DAD-QTOF system for the characterization of the phenolic profile of the argentinean plant Zuccagnia punctata and of the related propolis: New biomarkers. J. Func. Foods 2017, 33, 425–435. [Google Scholar] [CrossRef]
- Solorzano, E.R.; Di Gangi, I.M.; Roverso, M.; Favaro, G.; Bogialli, S.; Pastore, P. Low level of allergens in the Argentinean plant Zuccagnia punctata Cav.: Screening and Quality Control of North-Western Propolis Using an LC-DAD-QTOF System. Appl. Sci. 2019, 9, 3546. [Google Scholar] [CrossRef] [Green Version]
- González, M.; García, M.E.; Slanis, A.; Bonini, A.; Fiedler, S.; Fariña, L.; Dellacassa, E.; Condurso, C.; Lorenzo, D.; Russo, M.; et al. Phytochemical findings evidencing botanical origin of new propolis type from north-west Argentina. Chem. Biodivers. 2019, 16, e1800442. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; Tapia, A.; Luna., L.; Fabani, M.P.; Schmeda-Hirschmann, G.; Podio, N.S.; Wunderlin, D.A.; Feresin, G.E. Flavonoids, DPPH activity, and metal content allow determination of the geographical origin of propolis from the Province of San Juan (Argentina). J. Agric. Food Chem. 2009, 57, 2691–2698. [Google Scholar] [CrossRef]
- Lozina, L.; Peichoto, M.; Acosta, O.; Granero, G. Standarization and organoleptic and physicochemical characterization of 15 Argentinean Propolis. Lat. Am. J. Pharm. 2010, 29, 102–110. [Google Scholar]
- Isla, M.I.; Carrasco Juárez, B.; Nieva Moreno, M.I.; Zampini, I.; Ordóñez, R.; Sayago, J.; Cuello, S.; Alberto, M.R.; Bedescarrabure, E.; Alvarez, A.; et al. Effect of seasonal variations and collection form on antioxidant activity of propolis from San Juan, Argentina. J. Med. Food 2009, 12, 1334–1342. [Google Scholar] [CrossRef]
- Kumazawa, S.; Ahn, M.R.; Fujimoto, T.; Kato, M. Radical-scavenging activity and phenolic constituents of propolis from different regions of Argentina. Nat. Prod. Res. 2010, 24, 804–812. [Google Scholar] [CrossRef]
- Tosi, E.A.; Re, E.; Ortega, M.E.; Cazzoli, A.F. Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia Coli. Food Chem. 2007, 104, 1025–1029. [Google Scholar] [CrossRef]
- Busch, V.M.; Pereyra-Gonzalez, A.; Segatin, N.; Santagapita, P.R.; Ulrih, N.P.; Buera, M.P. Propolis encapsulation by spray drying: 1 characterization and stability. LWT 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Sánchez, M.; Luna, L.; Lima, B.; López, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav. (Zygophyllaceae). HPLC-MS and GC-MS characterization and antifungal activity. Food Chem. Toxicol. 2011, 49, 1970–1978. [Google Scholar]
- Mercado, M.I.; Moreno, M.A.; Ruiz, A.I.; Rodríguez, I.F.; Zampini, C.I.; Isla, M.I.; Ponessa, G.I. Morphoanatomical and histochemical characterization of Larrea species from Northwestern Argentina. Rev. Bras. Farmacogn. 2018, 28, 393–401. [Google Scholar] [CrossRef]
- Salas, A.; Mercado, M.I.; Zampini, I.C.; Ponessa, G.I.; Isla, M.I. Determination of botanical origin of propolis from Monte region of Argentina by histological and chemical methods. Nat. Prod. Commun. 2016, 11, 627–630. [Google Scholar] [CrossRef] [Green Version]
- Salas, A.S.; Mercado, M.I.; Orqueda, E.; Correa Uriburu, F.; García, M.E.; Pérez, J.; Alvarez, M.; Ponessa, G.; Maldonado, L.; Zampini, I.C.; et al. Zuccagnia-type Propolis from Argentina: A potential functional ingredient in food to pathologies associated to metabolic syndrome and oxidative stress. J. Food Sci. 2020, 85, 2578–2588. [Google Scholar] [CrossRef]
- Lersten, N.R.; Curtis, J.D. Survey of leaf anatomy, especially secretory structures, of tribe Caesalpinieae (Leguminosae, Caesalpinioideae). Plant Syst. Evol. 1996, 200, 1–39. [Google Scholar] [CrossRef]
- Mercado, M.I.; Ruiz, A.I.; Zampini, I.C.; Nuño, G.; Cuello, S.; Isla, M.I.; Ponessa, G.I. Arquitectura y morfoanatomía foliar y caulinar de Zuccagnia punctata (Fabaceae). Histolocalización de compuestos bioactivos. Lilloa 2013, 50, 58–68. [Google Scholar]
- Nieva Moreno, M.I.; Isla, M.I.; Cudmani, N.G.; Vattuone, M.A.; Sampietro, A.R. Screening of antibacterial activity of Amaicha del Valle (Tucumán, Argentina) propolis. J. Ethnopharmacol. 1999, 68, 97–102. [Google Scholar] [CrossRef]
- Salas, A.; Zampini, I.C.; Maldonado, L.; Isla, M.I. Development of a bioproduct for medicinal use with extracts of Zuccagnia-type propolis. Nat. Prod. Commun. 2018, 13, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Nieva Moreno, M.I.; Isla, M.I.; Vattuone, M.A.; Sampietro, A.R. Comparison of the free radical-scavenging activity of propolis from several regions. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Isla, M.I.; Nieva Moreno, M.I.; Vattuone, M.A.; Sampietro, A.R. Antioxidant activity of argentine propolis extracts. J. Etnopharmacol. 2001, 76, 165–170. [Google Scholar] [CrossRef]
- Oldoni, T.L.; Cabral, I.; D’Arce, M.; Rosalen, P.; Ikegaki, M.; Nascimento, A.; Alencar, S. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Tran, V.H.; Duke, R.; Abu-Mellal, A.; Duke, C. Propolis with high flavonoid content collected by honey bees from Acacia paradoxa. Phytochemistry 2012, 81, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.L.; Maboni, F.; Machado, G.; Alves, S.H.; de Vargas, A.C. Antimicrobial activity of propolis extract against Staphylococcus coagulase positive and Malassezia pachydermatis of canine otitis. Vet. Microbiol. 2010, 142, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Nuño, G.; Alberto, M.; Zampini, I.; Cuello, S.; Ordoñez, R.; Sayago, J.; Baroni, V.; Wunderlin, D.; Isla, M.I. The effect of Zuccagnia punctata Cav, an Argentina medicinal plant, on virulence factors from Candida species. Nat. Prod. Commun. 2014, 9, 933–936. [Google Scholar]
- Isla, M.I.; Moreno, A.; Nuño, G.; Carabajal, A.; Aberto, M.R.; Zampini, I.C. Zuccagnia punctata Cav.: A review of its traditional uses, phytochemistry, pharmacology and toxicology. Nat. Prod. Commun. 2016, 11, 1749–1755. [Google Scholar]
- Tangarife-Castaño, V.; Correa-Royero, J.; Zapata-Londoño, B.; Durán, C.; Stanshenko, E.; Mesa-Arango, A.C. Anti-Candida albicans activity, cytotoxicity and interaction with antifungal drugs of essential oils and extracts from aromatic and medicinal plants. Infection 2011, 11, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Carabajal, M.P.A.; Isla, M.I.; Zampini, I.C. Evaluation of antioxidant and antimutagenic activity of herbal teas from native plants used in traditional medicine in Argentina. South Afr. J. Bot. 2017, 110, 258–265. [Google Scholar] [CrossRef]
- Carabajal, M.P.A.; Isla, M.I.; Borsarelli, C.D.; Zampini, I.C. Influence of in vitro gastro-duodenal digestion on the antioxidant activity of single and mixed three “Jarilla” species infusions. J. Herb. Med. 2020, 19, 100296. [Google Scholar] [CrossRef]
- Carabajal, M.P.A.; Perea, M.C.; Isla, M.I.; Zampini, I.C. The use of jarilla native plants in a Diaguita-Calchaquí indigenous community from northwestern Argentina: An ethnobotanical, phytochemical and biological approach. J. Ethnopharmacol. 2020, 247, 112258. [Google Scholar] [CrossRef]
- Moreno, M.A.; Gómez-Mascaraque, L.; Arias, M.; Zampini, I.C.; Sayago, J.E.; Pino Ramos, L.L.; Schmeda-Hirschmann, G.; López-Rubio, A.; Isla, M.I. Electrosprayed chitosan microcapsules as delivery vehicles for vaginal phytoformulations. Carbohydr. Polym. 2018, 201, 425–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, V.; Bertolotti, S.G.; Criado, S.; Pappano, N.; Debattista, N.; García, N.A. Antioxidant properties of natural flavonoids: Quenching and generation of singlet molecular oxygen. J. Food Sci. Technol. 2001, 36, 25–33. [Google Scholar] [CrossRef]
- Morán Vieyra, F.; Boggetti, H.; Zampini, I.; Ordoñez, R.; Isla, M.; Alvarez, R.; De Rosso, V.; Mercadante, A.; Borsarelli, C. Singlet oxygen quenching and radical scavenging capacities of structurally related flavonoids present in Zuccagnia punctata Cav. Free Rad. Res. 2009, 43, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Wecksler, A.T.; Wagner, K.; Hammock, B.D. Rationally designed multitarget agents against inflammation and pain. Curr. Med. Chem. 2013, 20, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Antiinflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Kim, H.; Son, K.; Chang, H.; Kang, S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharm. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-κB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.R.; Nieva Moreno, M.I.; Zampini, I.C.; Isla, M.I. Anti-inflammatory activity of structurally related natural flavonoids. Bol. Latinoam. Caribe Plantas Med. Aromát. 2007, 6, 308–309. [Google Scholar]
- Alvarenga, L.; Cardozo, L.F.; Borges, N.A.; Chermut, T.R.; Ribeiro, M.; Leite, M.; Mafra, D. To bee or not to bee? The bee extract propolis as a bioactive compound in the burden of lifestyle disease. Nutrition 2020, 83, 111094. [Google Scholar] [CrossRef]
- Pastor-Villaescusa, B.; Sanchez Rodriguez, E.; Rangel-Huerta, O. Polyphenols in obesity and metabolic syndrome. Obesity 2018, 213–239. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.K.; Bharti, S.K. Therapeutic potential of chalcones as cardiovascular agents. Life Sci. 2016, 148, 154–172. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.Y.; Rao, L.; Rao, Y.; Guo, J.X.; Xiao, Z.Z.; Cao, J.Y.; Wang, B. Analogues of xanthone-chalcones and bis-chalcones as α-glucosidase inhibitors and anti-diabetes candidates. Eur. J. Med. Chem. 2017, 130, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bale, A.T.; Khan, K.M.; Salar, U.; Chigurupati, S.; Fasina, T.; Ali, F.; Perveen, S. Chalcones and bis-chalcones: As potential α-amylase inhibitors; synthesis, in vitro screening, and molecular modelling studies. Bioorg. Chem. 2018, 79, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Roco, J.; Alarcon, G.; Medina, M.; Zampini, C.; Isla, M.I.; Jerez, S. Beneficial effects of hydroalcoholic extract and flavonoids from Zuccagnia punctata in a rabbit model of vascular dysfunction induced by high cholesterol diet. Med. Chem. Res. 2017, 26, 1–9. [Google Scholar] [CrossRef]
- Roco, J.; Zampini, C.; Isla, M.I.; Jerez, S. Oral administration of Zuccagnia punctata extract improves lipid profile, reduces oxidative stress and normalizes vascular function in hypercholesterolemic rabbits. Phytomedicine 2018, 48, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordóñez, R.; Zampini, I.C.; Nieva Moreno, M.I.; Isla, M.I. Potential application of Argentine propolis to control some phytopathogenic bacteria. Microbiol. Res. 2011, 166, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Vallejo, A.M.; Ballester, A.R.; Zampini, C.; Isla, M.I.; Lopez-Rubio, A.; Fabra, M.J. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Zampini, I.C.; Villena, J.; Salva, S.; Herrera, M.; Isla, M.I.; Alvarez, S. Potentiality of standardized extract and isolated flavonoids from Zuccagnia punctata for the treatment of respiratory infections by Streptococcus pneumoniae: In vitro and in vivo studies. J. Ethnopharmacol. 2012, 140, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, S. Recent advances in role of propolis as natural additive in poultry nutrition. J. Apic. Sci. 2017, 61, 2. [Google Scholar] [CrossRef] [Green Version]
- Bankova, V.; Popova, M.; Trusheva, B. New emerging fields of application of propolis. Maced. J. Chem. Chem. Eng. 2016, 35, 1–11. [Google Scholar] [CrossRef]


| Phenolic Components | SE | T | CH | S | J | C | RN | LP | ER | SJ | M | SF | BA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acid and derivates | |||||||||||||
| Coumaric acid | X | X | X | X | X | X | X | ND | X | X | X | X | |
| Caffeic acid | ND | ND | ND | ND | ND | ND | X | ND | ND | X | X | X | |
| Ferulic acid | X | X | X | X | ND | X | X | ND | X | X | ND | X | |
| Cinnamic acid | X | X | X | ND | ND | X | ND | X | X | ND | ND | ND | |
| CAPE | X | X | X | ND | ND | X | X | ND | X | X | ND | ND | |
| Flavanones | |||||||||||||
| Pinobanksin | X | X | X | ND | ND | X | X | ND | X | X | X | ND | ND |
| Pinocembrin | X | X | X | ND | ND | X | X | ND | X | X | X | ND | X |
| Pinocembrin derivate | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| Naringenin | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | X |
| 7-hydroxy-8-methoxyflavanone | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| Flavone | |||||||||||||
| Apigenin | X | X | X | X | X | X | X | X | X | X | ND | X | |
| Chrysin | X | X | X | ND | ND | X | X | X | X | X | X | X | X |
| Tectochrysin | X | X | X | ND | ND | X | X | ND | X | X | X | ND | ND |
| Flavonol | |||||||||||||
| Galangin | X | X | X | ND | ND | X | X | ND | X | X | X | X | X |
| Quercetin | X | X | X | ND | ND | X | ND | ND | X | ND | X | X | |
| Kaempferol | X | X | X | ND | ND | X | ND | ND | X | ND | ND | ND | |
| Kaempferide | X | X | X | ND | ND | X | X | ND | X | X | ND | ND | |
| Lignans | |||||||||||||
| 3′methyl-nordihydroguaiaretic acid (MNDGA) | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| nordihydroguaiaretic acid(NDGA) | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| 3 (4-[4-(4-hydroxy-phenyl)-2,3-dimethyl-butyl]-benzene-1,2-diol) | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| meso-(rel 7S,8S,7′R,8′R)-3,4,3′,4′-tetrahydroxy7,7′-epoxylignan | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| 5 (7S,8S,7′S,8′S)-3,3′,4′-trihy droxy-4-methoxy-7,7′-epoxylignan | ND | ND | ND | ND | ND | ND | ND | ND | ND | X | ND | ND | ND |
| Compounds | Geographical Sites | References |
|---|---|---|
| Flavanones | ||
| 7-hydroxyflavanone | Tucumán, Catamarca | Agüero et al., 2010; Solorzano et al., 2012; Solórzano et al., 2017 |
| 7,8-dihydroxyflavanone | Catamarca. Tucumán | Solórzano et al., 2017 |
| 3,7-dihydroxyflavanone | Catamarca. Tucumán | Solórzano et al., 2017 |
| 4′,7-dihydroxyflavanone (liquiritigenin) | Tucumán | Salas et al., 2016a |
| 5,7-dihydroxyflavanone (pinocembrin) | Catamarca, Tucumán | Agüero et al., 2010, Solórzano et al., 2017 |
| 3,5,7-trihydroxyflavanone (pinobanksin) | Catamarca | Solorzano et al., 2012 |
| 3,7,8-trihydroxydihydroflavanone | Catamarca, Tucumán | Solórzano et al., 2017 |
| 5-hydroxy- 7- methoxyflavanone (pinostrobin) | Tucumán, Catamarca | Agüero et al., 2010, Solorzano et al., 2012 |
| 7-hydroxy- 8-methoxyflavanone | Catamarca, Tucumán | Vera et al., 2011; Agüero et al., 2010; Solorzano et al., 2012; Solórzano et al., 2017 |
| 7,4′-dihydroxy-5-methoxyflavanone | Catamarca, Tucumán | Vera et al., 2011, Agüero et al., 2010; Solorzano et al., 2017 |
| 3β, 7-dihydroxy-5-methoxyflavanone | Catamarca | Vera et al., 2011 |
| 7-hydroxy-5,8-dimethoxyflavanone | Catamarca | Vera et al., 2011 |
| pinobanksin-5-methyl ether (3,7-dihydroxy-5-methoxyflavanone) | Catamarca, Tucumán | Solórzano et al., 2017 |
| Flavones | ||
| 5,7-dihydroxyflavone (chrysin) | Catamarca, Tucumán | Solorzano et al., 2012; Solórzano et al., 2017 |
| 3,7-dihydroxyflavone | Catamarca, Tucumán | Solórzano et al., 2017 |
| 3,5,7-trihydroxyflavone (galangin) | Catamarca, Tucumán | Agüero et al., 2010, Vera et al., 2011; Solórzano et al., 2017 |
| 3-hydroxy-7,8-dimethoxyflavone | Tucumán | Agüero et al., 2010, Vera et al., 2011 |
| 7-hydroxy-5,8 dimethoxyflavone | Catamarca | Vera et al., 2011 |
| 5-hydroxy-4′,7-dimethoxyflavone | Catamarca | Vera et al., 2011 |
| 3,7-dihydroxy-8-methoxyflavone | Catamarca, Tucumán | Vera et al., 2011; Solorzano et al., 2012, Solórzano et al., 2017 |
| 3,5-dihydroxy-7-methoxyflavone (izalpinin) | Tucumán | Agüero et al., 2010 |
| 3,5-dihydroxy-7,8-dimethoxyflavone | Catamarca, Tucumán | Vera et al., 2011; Solorzano et al., 2012; Solórzano et al., 2017 |
| 4′, 5-dihydroxy-3,7,8-trimethoxyflavone | Catamarca | Vera et al., 2011 |
| 3,4′,5-trihydroxy-7-methoxyflavone (rhamnocitrin) | Catamarca, Tucumán | Agüero et al., 2010; Solórzano et al., 2017 |
| Chalcones | ||
| 2′,4′-dihydroxychalcone (DHC) | Catamarca, Tucumán | Vera et al., 2011; Agüero et al., 2010; Solorzano et al., 2012, Salas et al., 2016a,b; Solórzano et al., 2017 |
| 2′,4′-dihydroxydihydrochalcone | Catamarca, Tucumán | Solórzano et al., 2017 |
| 4′-hydroxy-2′-methoxydihydrochalcone | Catamarca, Tucumán | Solórzano et al., 2017 |
| 2′,4′-dihydroxy-3′-methoxychalcone (DHMC) | Catamarca, Tucumán | Vera et al., 2011; Agüero et al., 2010; Solorzano et al., 2012; Salas et al., 2016a,b; Solórzano et al., 2017 |
| 2′,4′,4-trihydroxy-6′-methoxychalcone | Catamarca, Tucumán | Vera et al., 2011, Solórzano et al., 2017 |
| Phenolic acids and esters | ||
| cinnamic acid | Tucumán | Salas et al., 2016b |
| 1,1-dimethylallyl caffeic acid | Catamarca, Tucumán | Solórzano et al., 2017 |
| caffeoyl dihydrocaffeate | Tucumán | Salas et al., 2016b |
| geranyl caffeate | Catamarca, Tucumán | Solórzano et al., 2019 |
| pentenyl caffeate | Catamarca, Tucumán | Solórzano et al., 2019 |
| benzyl caffeate | Catamarca, Tucumán | Solórzano et al., 2019 |
| cinnamyl caffeate | Catamarca, Tucumán | Solórzano et al., 2019 |
| methyl caffeate | Catamarca | Solórzano et al., 2019 |
| caffeic acid prenyl ester | Tucumán | Salas et al., 2016b |
| 3,4-dihydroxyphenethyl caffeic acid ester (teucrol) | Tucumán | Salas et al., 2016b |
| 1-methyl-3-(4-hydroxyphenyl)-propyl caffeic acid ester | Catamarca | Solórzano et al., 2017 |
| 1-methyl-3-(4 -hydroxyphenyl)-propyl p-coumaric acid ester | Catamarca | Solórzano et al., 2017 |
| 1-methyl-3-(3′,4′-dihydroxyphenyl)-propyl caffeic acid ester | Catamarca | Solórzano et al., 2017 |
| 4′-terbutyloxyphenyl p-coumaric acid ester | Catamarca, Tucumán | Solórzano et al., 2017 |
| 1-methyl-3-(4′-hydroxyphenyl)-propyl p-coumaric acid ester | Catamarca, Tucumán | Solórzano et al., 2017 |
| 3,7-dimethyl-2,6-octadienyl caffeic acid ester (geranyl caffeate) | Catamarca, Tucumán | Solórzano et al., 2017 |
| 1-methyl-3-(3′,4′-dihydroxyphenyl)-propyl ferulic acid ester | Catamarca, Tucumán | Solórzano et al., 2017 |
| 2-methyl-3-(3′-hydroxy-4′-methoxyphenyl)-propyl caffeic acid ester | Catamarca, Tucumán | Solórzano et al., 2017 |
| Volatile compounds | ||
| trans-linalool oxide (furanoid) | Tucumán | Gonzalez et al., 2019 |
| cis-linalool oxide (furanoid) | Tucumán | Gonzalez et al., 2019 |
| (E)-anethole | Tucumán | Gonzalez et al., 2019 |
| Linalool | Tucumán | Gonzalez et al., 2019 |
| cis-linalyl oxide (pyranoid) | Tucumán | Gonzalez et al., 2019 |
| p-cymen-8-ol | Tucumán | Gonzalez et al., 2019 |
| 2,3,6-trimethylbenzaldehyde | Tucumán | Gonzalez et al., 2019 |
| Chrysanthenone | Tucumán | Gonzalez et al., 2019 |
| p-mentha-1,5-dien-8-ol | Tucumán | Gonzalez et al., 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampini, I.C.; Salas, A.L.; Maldonado, L.M.; Simirgiotis, M.J.; Isla, M.I. Propolis from the Monte Region in Argentina: A Potential Phytotherapic and Food Functional Ingredient. Metabolites 2021, 11, 76. https://doi.org/10.3390/metabo11020076
Zampini IC, Salas AL, Maldonado LM, Simirgiotis MJ, Isla MI. Propolis from the Monte Region in Argentina: A Potential Phytotherapic and Food Functional Ingredient. Metabolites. 2021; 11(2):76. https://doi.org/10.3390/metabo11020076
Chicago/Turabian StyleZampini, Iris Catiana, Ana Lia Salas, Luis M. Maldonado, Mario J. Simirgiotis, and María Inés Isla. 2021. "Propolis from the Monte Region in Argentina: A Potential Phytotherapic and Food Functional Ingredient" Metabolites 11, no. 2: 76. https://doi.org/10.3390/metabo11020076
APA StyleZampini, I. C., Salas, A. L., Maldonado, L. M., Simirgiotis, M. J., & Isla, M. I. (2021). Propolis from the Monte Region in Argentina: A Potential Phytotherapic and Food Functional Ingredient. Metabolites, 11(2), 76. https://doi.org/10.3390/metabo11020076