Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-O Analysis of Intermuscular Fat Aroma
2.2. Quantification of Odorants Using the Stable Isotope Dilution Assay (SIDA)
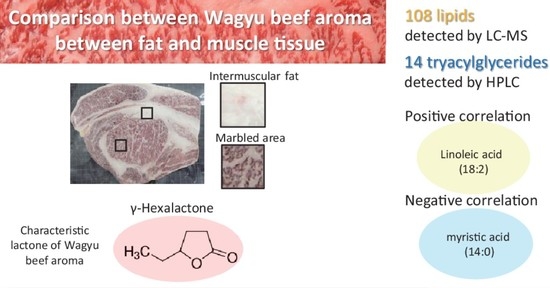
2.3. Lipidomic Analysis of Japanese Black Cattle Meat by Liquid Chromatography-Mass Spectrometry (LC-MS)
2.4. Quantitative Analysis of the Fatty Acid and TAG Molecular Species Compositions and Odorants
2.5. Correlation between the Composition of Lipids and Odorants Related to Wagyu Beef Aroma
3. Materials and Methods
3.1. Sample Collection
3.2. GC-O Analysis of the Odorant Concentrations in Boiled Beef
3.3. Quantification of Lactone
3.4. LC-MS Analysis
3.5. Analysis of Fatty Acids and TAG Composition
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat. Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Matsuishi, M.; Fujimori, M.; Okitani, A. Wagyu Beef Aroma in Wagyu (Japanese Black Cattle) Beef Preferred by the Japanese over Imported Beef. Nihon Chikusan Gakkaiho 2001, 72, 498–504. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. Fems Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef] [Green Version]
- Shirouchi, B.; Albrecht, E.; Nuernberg, G.; Maak, S.; Olavanh, S.; Nakamura, Y.; Sato, M.; Gotoh, T.; Nuernberg, K. Fatty acid profiles and adipogenic gene expression of various fat depots in Japanese Black and Holstein steers. Meat. Sci. 2014, 96, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yamamoto, I.; Jeong, J.S.; Nade, T.; Arai, T.; Kimura, N. Relationship between adipose maturity and fatty acid composition in various adipose tissues of Japanese Black, Holstein and Crossbred (F1) steers. Anim. Sci. J. 2011, 82, 689–697. [Google Scholar] [CrossRef]
- Shahrai, N.N.; Babji, A.S.; Maskat, M.Y.; Razali, A.F.; Yusop, S.M. Effects of marbling on physical and sensory characteristics of ribeye steaks from four different cattle breeds. Asian Australas. J. Anim. Sci. 2020. [Google Scholar] [CrossRef]
- Watanabe, G.; Motoyama, M.; Orita, K.; Takita, K.; Aonuma, T.; Nakajima, I.; Tajima, A.; Abe, A.; Sasaki, K. Assessment of the dynamics of sensory perception of Wagyu beef strip loin prepared with different cooking methods and fattening periods using the temporal dominance of sensations. Food Sci. Nutr. 2019, 7, 3538–3548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Guo, Y.; Du, W.; Zhang, X.; Li, A.; Miao, X. Global transcriptome analysis identifies differentially expressed genes related to lipid metabolism in Wagyu and Holstein cattle. Sci. Rep. 2017, 7, 5278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawaguchi, F.; Kigoshi, H.; Fukushima, M.; Iwamoto, E.; Kobayashi, E.; Oyama, K.; Mannen, H.; Sasazaki, S. Whole-genome resequencing to identify candidate genes for the QTL for oleic acid percentage in Japanese Black cattle. Anim. Sci. J. 2019, 90, 467–472. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Xu, L.; Zhu, B.; Liu, Y.; Bordbar, F.; Chen, Y.; Zhang, L.; Gao, X.; Gao, H.; et al. Genome-Wide Scan Identifies Selection Signatures in Chinese Wagyu Cattle Using a High-Density SNP Array. Animals 2019, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Characteristic Aroma of Beef from Japanese Black Cattle (Japanese Wagyu) via Sensory Evaluation and Gas Chromatography-Olfactometry. Metabolites 2021, 11, 56. [Google Scholar] [CrossRef]
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Amano, Y.; Kumazawa, K. Identification and Characterization of Volatile Components Causing the Characteristic Flavor of Wagyu Beef (Japanese Black Cattle). J. Agric. Food Chem. 2017, 65, 8691–8695. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Tago, A.; Yoshinaga-Kiriake, A.; Gotoh, N. Characterization of lactones in Wagyu (Japanese beef) and imported beef by combining solvent extraction and gas chromatography–mass spectrometry. LWT 2021, 135, 110015. [Google Scholar] [CrossRef]
- Kishimoto, T.; Noba, S.; Yako, N.; Kobayashi, M.; Watanabe, T. Simulation of Pilsner-type beer aroma using 76 odor-active compounds. J. Biosci. Bioeng. 2018, 126, 330–338. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Ferreira, V. Gas Chromatography Olfactometry (GC-O) for the (Semi)Quantitative Screening of Wine Aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Iwamoto, E.; Kato, Y.; Shinohara, M.; Shirai, Y.; Yamanoue, M. Comparative metabolomics of Japanese Black cattle beef and other meats using gas chromatography-mass spectrometry. Biosci. Biotechnol. Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, C.N.; Ko, K.B.; Park, S.P.; Kim, H.K.; Kim, J.M.; Ryu, Y.C. Comparisons of Beef Fatty Acid and Amino Acid Characteristics between Jeju Black Cattle, Hanwoo, and Wagyu Breeds. Food Sci. Anim. Resour. 2019, 39, 402–409. [Google Scholar] [CrossRef]
- Xie, Y.R.; Busboom, J.R.; Cornforth, D.P.; Shenton, H.T.; Gaskins, C.T.; Johnson, K.A.; Reeves, J.J.; Wright, R.W.; Cronrath, J.D. Effects of time on feed and post-mortem aging on palatability and lipid composition of crossbred Wagyu beef. Meat. Sci. 1996, 43, 157–166. [Google Scholar] [CrossRef]
- Dias, L.G.; Duarte, G.H.B.; Mariutti, L.R.B.; Bragagnolo, N. Aroma profile of rice varieties by a novel SPME method able to maximize 2-acetyl-1-pyrroline and minimize hexanal extraction. Food Res. Int. 2019, 123, 550–558. [Google Scholar] [CrossRef]
- Wei, X.; Handoko, D.D.; Pather, L.; Methven, L.; Elmore, J.S. Evaluation of 2-acetyl-1-pyrroline in foods, with an emphasis on rice flavour. Food Chem. 2017, 232, 531–544. [Google Scholar] [CrossRef]
- Young, O.A.; Baumeister, B.M.B. The effect of diet on the flavour of cooked beef and the odour compounds in beef fat. N. Z. J. Agric. Res. 1999, 42, 297–304. [Google Scholar] [CrossRef]
- San-Juan, F.; Cacho, J.; Ferreira, V.; Escudero, A. 3-Methyl-2-butene-1-thiol: Identification, analysis, occurrence and sensory role of an uncommon thiol in wine. Talanta 2012, 99, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tang, Q.; Hayat, K.; Karangwa, E.; Zhang, X.; Xiao, Z. Effect of enzymatic hydrolysis with subsequent mild thermal oxidation of tallow on precursor formation and sensory profiles of beef flavours assessed by partial least squares regression. Meat. Sci. 2014, 96. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging–A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-Analysis of the Core Aroma Components of Grape and Wine Aroma. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Toda, Y.; Nakagita, T.; Hirokawa, T.; Yamashita, Y.; Nakajima, A.; Narukawa, M.; Ishimaru, Y.; Uchida, R.; Misaka, T. Positive/Negative Allosteric Modulation Switching in an Umami Taste Receptor (T1R1/T1R3) by a Natural Flavor Compound, Methional. Sci. Rep. 2018, 8, 11796. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Muthumalage, T.; Rahman, I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 2018, 288, 143–155. [Google Scholar] [CrossRef]
- Resconi, V.C.; Escudero, A.; Campo, M.M. The development of aromas in ruminant meat. Molecules 2013, 18, 6748–6781. [Google Scholar] [CrossRef] [Green Version]
- Wanikawa, A.; Hosoi, K.; Takise, I.; Kato, T. Detection or γ-Lactones in Malt Whisky. J. Inst. Brew. 2000, 106, 39–44. [Google Scholar] [CrossRef]
- Xin, R.; Liu, X.; Wei, C.; Yang, C.; Liu, H.; Cao, X.; Wu, D.; Zhang, B.; Chen, K. E-Nose and GC-MS Reveal a Difference in the Volatile Profiles of White- and Red-Fleshed Peach Fruit. Sensors 2018, 18, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyquist, K.M.; O’Quinn, T.G.; Drey, L.N.; Lucherk, L.W.; Brooks, J.C.; Miller, M.F.; Legako, J.F. Palatability of beef chuck, loin, and round muscles from three USDA quality grades. J. Anim Sci. 2018, 96, 4276–4292. [Google Scholar] [CrossRef] [PubMed]
- Iida, F.; Saitou, K.; Kawamura, T.; Yamaguchi, S.; Nishimura, T. Effect of fat content on sensory characteristics of marbled beef from Japanese Black steers. Anim. Sci. J. 2015, 86, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Reis, M.G.; Subbaraj, A.K.; Cameron-Smith, D.; Fraser, K.; Jonker, A.; Craigie, C.R. Distribution of fatty acids and phospholipids in different table cuts and co-products from New Zealand pasture-fed Wagyu-dairy cross beef cattle. Meat. Sci. 2018, 140, 26–37. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. The assembly of lipid droplets and their roles in challenged cells. Embo. J. 2018, 37, e98947. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Kaczmarska, K.; Paterson, J.; Piyasiri, U.; Warner, R. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat. Sci. 2017, 133, 61–68. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Zhang, Y.; Shen, Y.; Su, H.; Jin, J.; Jin, Q.; Wang, X. Characterization of Positional Distribution of Fatty Acids and Triacylglycerol Molecular Compositions of Marine Fish Oils Rich in Omega-3 Polyunsaturated Fatty Acids. Biomed. Res. Int. 2018, 2018, 3529682. [Google Scholar] [CrossRef]
- Silva, R.; Aguiar, T.Q.; Coelho, E.; Jiménez, A.; Revuelta, J.L.; Domingues, L. Metabolic engineering of Ashbya gossypii for deciphering the de novo biosynthesis of γ-lactones. Microb. Cell Fact. 2019, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- Kobe Beef Marketing & Distribution Promotion Association. Kobe Beef. 2020. Available online: http://www.kobe-niku.jp/top.html (accessed on 25 March 2021).
- Namekawa, J.; Yasui, M.; Katayanagi, A.; Shirai, M.; Asai, F. Increased hepatic triglyceride level induced by a glucokinase activator in mice. Fundam. Toxicol. Sci. 2018, 5, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. A simple procedure for rapid transmethylation of glycerolipids and cholesteryl esters. J. Lipid. Res. 1982, 23, 1072–1075. [Google Scholar] [CrossRef]




| Intermuscular Fat | Odor Quality c | FD Factor b (Log4) | Relative Value (Type B/Holstein) | ||||
|---|---|---|---|---|---|---|---|
| Japanese Black Cattle | |||||||
| No. | RI a | Conpound | Type A | Type B | Holstein | ||
| 1 | 983 | 2,3-Butanedione | Buttery | 3 | 3 | 3 | 1.0 |
| 2 | 1105 | Hexanal | Fresh leaves | 2 | 2 | 3 | 0.7 |
| 3 | 1123 | 3-Methyl-2-butene-1-thiol | Burnt | 1 | 2 | 0 | - |
| 4 | 1291 | Octanal | Green fresh | 1 | 2 | 0 | - |
| 5 | 1299 | 2-Methyl-3-furanthiol | Nuts | 7 | 7 | 7 | 1.0 |
| 6 | 1331 | 2-Acetyl-1-pyrroline | Grain | 0.3 | 5 | 0 | - |
| 7 | 1371 | 1,5-Octadien-3-one | Green, Metallic | 4 | 5 | 4 | 1.3 |
| 8 | 1423 | Nonanal | Oil oxidation | 7 | 7 | 7 | 1.0 |
| 9 | 1438 | (E)-2-Octenal | Grassy-smelling | 6 | 6 | 6 | 1.0 |
| 10 | 1441 | Methional | Stewed potatoes | 0 | 5 | 0 | - |
| 11 | 1501 | Decanal | Green fresh | 7 | 7 | 7 | 1.0 |
| 12 | 1530 | (E)-2-Nonenal | Oil oxidation | 7 | 7 | 7 | 1.0 |
| 13 | 1577 | (2E,6Z)-Nona-2,6-dienal | Cucumber | 4 | 5 | 4 | 1.3 |
| 14 | 1623 | Butyric acid | Cheese odor | 4 | 4 | 4 | 1.0 |
| 15 | 1669 | Isovaleric acid | Cheese odor | 1 | 1 | 1 | 1.0 |
| 16 | 1684 | γ-Hexalactone | Sweet, milk. | 0 | 0 | 0 | ND d |
| 17 | 1690 | (2E,4E)-2,4-Nonadienal | Oil oxidation | 7 | 7 | 6 | 1.2 |
| 18 | 1731 | 2-Acetyl-1-thiazoline | Grain | 2 | 1 | 0.3 | 3.3 |
| 19 | 1757 | 2-Undecenal | Oil oxidation | 2 | 4 | 2 | 2.0 |
| 20 | 1787 | γ-Heptalactone | Sweet, milk. | 0 | 0 | 0 | ND |
| 21 | 1800 | (E,E)-2,4-Decadienal | Oil oxidation | 7 | 7 | 7 | 1.0 |
| 22 | 1842 | Hexanoic acid | Dust cloth | 2 | 2 | 2 | 1.0 |
| 23 | 1890 | γ-Octalactone | Lactone Sweet Scent | 5 | 6 | 0.3 | 20.0 |
| 24 | 1928 | β-Ionone | Violet | 0.3 | 2 | 0.3 | 6.7 |
| 25 | 1941 | Maltol | Sweet yogurt | 0.3 | 0.3 | 0.3 | 1.0 |
| 26 | 1989 | 4,5-Epoxy-2(E)-decenal | Metal | 7 | 7 | 7 | 1.0 |
| 27 | 2004 | γ-Nonalactone | Lactone Sweet Scent | 1 | 5 | 0.3 | 16.7 |
| 28 | 2021 | Franeol | Sweet yogurt | 0 | 5 | 0 | - |
| 29 | 2099 | γ-Decalactone | Lactone Sweet Scent | 0.3 | 0.3 | 0.3 | 1.0 |
| 30 | 2171 | 4-Vinyl guaiacol | Smoky | 5 | 5 | 5 | 1.0 |
| 31 | 2185 | δ-Decalactone | Sweet Lactones | 3 | 5 | 0.3 | 16.7 |
| 32 | 2188 | 2-Aminoacetopheone | Grape | 2 | 3 | 3 | 1.0 |
| 33 | 2256 | 4-Vinyl phenol | Smoky | 3 | 5 | 4 | 1.3 |
| 34 | 2288 | Decanoic acid | Dust cloth | 2 | 2 | 1 | 2.0 |
| 35 | 2361 | 9-Decenoic acid | Dust cloth | 2 | 3 | 2 | 1.5 |
| 36 | 2368 | Indole | indole | 4 | 3 | 0 | - |
| 37 | 2445 | 3-Methoxyphenol | Vanilla | 0 | 0 | 0 | ND |
| 38 | 2459 | 3-Methylindole | Indole | 4 | 3 | 0 | - |
| 39 | 2537 | Vanillin | Chocolate, vanilla | 4 | 5 | 5 | 1.0 |
| Triacylglyceride (%) | Muscle Tissue | |||
|---|---|---|---|---|
| POO a | 29.9 | ± | 1.7 | |
| POP b | 9.8 | ± | 1.1 | |
| PPoO c | 8.2 | ± | 1.0 | |
| POS | 7.9 | ± | 1.5 | |
| SOO | 7.3 | ± | 1.1 | |
| OOO | 7.2 | ± | 1.6 | |
| MOP d | 4.9 | ± | 0.7 | |
| OOPo | 4.0 | ± | 1.2 | |
| PLO | 3.1 | ± | 0.6 | |
| PPP | 2.9 | ± | 0.4 | |
| SOS | 1.7 | ± | 0.5 | |
| PPS | 1.3 | ± | 0.3 | |
| POMa | 1.3 | ± | 0.2 | |
| SSS | 0.1 | ± | 0.1 | |
| Unknown e | 10.2 | ± | 1.7 | |
| Fatty Acid (%) | Symbol | Muscle Tissue | ||
| C18:1 | O | 48.8 | ± | 2.2 |
| C16:0 | P | 24.9 | ± | 1.9 |
| C18:0 | S | 9.7 | ± | 1.4 |
| C16:1 | Po | 4.5 | ± | 0.9 |
| C14:0 | M | 2.6 | ± | 0.5 |
| C18:2 | L | 2.6 | ± | 0.6 |
| C14:1 | Mo | 1.1 | ± | 0.3 |
| C17:0 | Ma | 0.8 | ± | 0.2 |
| C15:0 | Pe | 0.3 | ± | 0.1 |
| C18:3 | Al | 0.3 | ± | 0.1 |
| Other e | - | 4.4 | ± | 0.5 |
| LACTONES (ng/g Beef) | Muscle Tissue | |||
| γ-hexalactone | 2.6 | ± | 0.9 | |
| γ-heptalactone | 0.3 | ± | 0.1 | |
| γ-decalactone | 2.1 | ± | 0.8 | |
| γ-octalactone | 4.0 | ± | 4.4 | |
| γ-nonalactone | 2.5 | ± | 0.7 | |
| δ-decalactone | 8.5 | ± | 3.8 | |
| γ-undecalactone | 4.5 | ± | 4.4 | |
| Muscle Tissue | POO a | POP b | PPoO c | POS | SOO | OOO | MOP d | OOPo | PLO | PPP | SOS | PPS | POMa | SSS | Unknown e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| γ-hexalactone | 0.20 | −0.38 | −0.31 | 0.11 | 0.31 | 0.24 | −0.50 | 0.10 | 0.40 | −0.22 | 0.26 | −0.04 | −0.11 | −0.18 | −0.25 |
| γ-heptalactone | 0.36 | −0.30 | −0.42 | 0.17 | 0.29 | 0.04 | −0.39 | −0.06 | 0.35 | −0.01 | 0.24 | −0.12 | 0.02 | −0.11 | −0.29 |
| γ-octalactone | −0.30 | 0.09 | 0.16 | −0.15 | −0.30 | −0.02 | 0.22 | 0.20 | −0.05 | 0.12 | −0.20 | 0.00 | 0.13 | −0.30 | 0.31 |
| γ-nonalactone | 0.01 | −0.02 | 0.20 | −0.26 | −0.18 | 0.08 | 0.11 | 0.17 | 0.21 | −0.24 | −0.30 | −0.30 | −0.41 | −0.36 | 0.19 |
| γ-decalactone | −0.26 | 0.23 | 0.42 | −0.31 | −0.43 | −0.03 | 0.38 | 0.22 | 0.03 | −0.19 | −0.43 | −0.20 | −0.44 | −0.37 | 0.41 |
| δ-decalactone | 0.06 | 0.28 | 0.00 | 0.12 | −0.21 | −0.32 | 0.21 | −0.20 | 0.00 | 0.29 | −0.09 | 0.08 | 0.14 | −0.26 | 0.05 |
| γ-undecalactone | 0.21 | 0.29 | −0.08 | 0.16 | −0.13 | −0.36 | 0.19 | −0.25 | −0.12 | 0.21 | −0.01 | 0.13 | 0.03 | 0.01 | −0.05 |
| POO a | POP b | PPoO c | POS | SOO | OOO | MOP d | OOPo | PLO | PPP | SOS | PPS | POMa | SSS | Unknown e | |
| C14:0 | −0.62 | 0.77 | 0.52 | −0.16 | −0.77 | −0.45 | 0.98 | −0.10 | −0.25 | 0.49 | −0.51 | 0.28 | 0.25 | −0.30 | 0.60 |
| C14:1 | −0.84 | 0.03 | 0.79 | −0.74 | −0.64 | 0.37 | 0.53 | 0.67 | −0.12 | −0.29 | −0.73 | −0.35 | −0.39 | −0.32 | 0.90 |
| C15:0 | −0.31 | 0.49 | 0.11 | 0.10 | −0.59 | −0.53 | 0.62 | −0.30 | −0.02 | 0.83 | −0.26 | 0.39 | 0.79 | −0.29 | 0.36 |
| C16:0 | −0.05 | 0.97 | −0.06 | 0.47 | −0.55 | −0.90 | 0.71 | −0.67 | −0.23 | 0.74 | 0.00 | 0.76 | 0.37 | −0.15 | 0.09 |
| C16:1 | −0.87 | −0.11 | 0.92 | −0.88 | −0.61 | 0.51 | 0.49 | 0.80 | −0.08 | −0.33 | −0.84 | −0.53 | −0.29 | −0.36 | 0.89 |
| C17:0 | 0.13 | 0.29 | −0.36 | 0.44 | −0.12 | −0.55 | 0.18 | −0.51 | 0.07 | 0.83 | 0.18 | 0.43 | 0.88 | −0.02 | −0.13 |
| C18:0 | 0.66 | 0.15 | −0.94 | 0.92 | 0.71 | −0.47 | −0.48 | −0.76 | −0.17 | 0.30 | 0.97 | 0.61 | 0.21 | 0.54 | −0.83 |
| C18:1 | 0.11 | −0.88 | 0.12 | −0.47 | 0.51 | 0.91 | −0.69 | 0.65 | −0.01 | −0.84 | −0.02 | −0.73 | −0.46 | 0.17 | −0.11 |
| C18:2 | 0.20 | −0.52 | 0.10 | −0.32 | 0.05 | 0.32 | −0.34 | 0.34 | 0.93 | −0.35 | −0.22 | −0.48 | −0.22 | −0.23 | −0.08 |
| C18:3 | 0.50 | −0.58 | −0.26 | 0.01 | 0.42 | 0.24 | −0.57 | 0.02 | 0.57 | −0.15 | 0.15 | −0.27 | 0.16 | −0.05 | −0.42 |
| Other | 0.26 | −0.40 | −0.25 | 0.02 | 0.17 | 0.05 | −0.32 | 0.01 | 0.69 | 0.14 | 0.05 | −0.22 | 0.28 | −0.20 | −0.18 |
| C14:0 | C14:1 | C15:0 | C16:0 | C16:1 | C17:0 | C18:0 | C18:1 | C18:2 | C18:3 | Other e | |||||
| γ-hexalactone | −0.53 | −0.22 | −0.21 | −0.34 | −0.28 | −0.10 | 0.21 | 0.22 | 0.54 | 0.41 | 0.31 | ||||
| γ-heptalactone | −0.39 | −0.33 | 0.01 | −0.22 | −0.41 | 0.25 | 0.26 | 0.09 | 0.37 | 0.40 | 0.51 | ||||
| γ-octalactone | 0.23 | 0.24 | 0.18 | 0.12 | 0.18 | 0.07 | −0.21 | −0.12 | −0.03 | −0.24 | −0.01 | ||||
| γ-nonalactone | 0.09 | 0.30 | −0.08 | 0.03 | 0.11 | −0.21 | −0.25 | 0.02 | 0.15 | −0.08 | 0.04 | ||||
| γ-decalactone | 0.35 | 0.54 | −0.09 | 0.24 | 0.36 | −0.33 | −0.37 | −0.14 | −0.07 | −0.49 | −0.24 | ||||
| δ-decalactone | 0.13 | −0.07 | 0.25 | 0.31 | −0.07 | 0.20 | 0.03 | −0.32 | −0.08 | −0.09 | 0.16 | ||||
| γ-undecalactone | 0.20 | −0.07 | 0.08 | 0.35 | −0.16 | 0.18 | 0.07 | −0.24 | −0.24 | −0.22 | −0.03 | ||||
| Analysis of Lactone, Fatty Acid, and TAG Composition in Marbled Area | |||||
| Group | Position | Meat Quality Grade | Number of Cattle | Slaughtered Age | Gender |
| (Month) | |||||
| Type A | Musculus longissimus | ≥ A4 | 10 | 29.5 ± 0.9 | Steer |
| Type B | Musculus longissimus | ≥ A4 | 10 | 32.3 ± 1.3 | Steer |
| Analysis of LC-MS | |||||
| Group | Position | Meat Quality Grade | Number of Cattle | Slaughtered Age | Gender |
| (Month) | |||||
| Type A | Musculus longissimus | A3 | 3 | 28.2 ± 0.3 | Steer |
| A5 | 3 | 28.1 ± 0.5 | Steer | ||
| Adductor magnus | A3 | 3 | 28.2 ± 0.3 | Steer | |
| A5 | 3 | 28.1 ± 0.5 | Steer | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, S.; Sasaki, R.; Nakabayashi, R.; Yamanoue, M.; Sirai, Y.; Iwamoto, E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites 2021, 11, 203. https://doi.org/10.3390/metabo11040203
Ueda S, Sasaki R, Nakabayashi R, Yamanoue M, Sirai Y, Iwamoto E. Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites. 2021; 11(4):203. https://doi.org/10.3390/metabo11040203
Chicago/Turabian StyleUeda, Shuji, Ryo Sasaki, Rio Nakabayashi, Minoru Yamanoue, Yasuhito Sirai, and Eiji Iwamoto. 2021. "Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle" Metabolites 11, no. 4: 203. https://doi.org/10.3390/metabo11040203
APA StyleUeda, S., Sasaki, R., Nakabayashi, R., Yamanoue, M., Sirai, Y., & Iwamoto, E. (2021). Exploring the Lipids Involved in the Formation of Characteristic Lactones in Japanese Black Cattle. Metabolites, 11(4), 203. https://doi.org/10.3390/metabo11040203