An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus ilex at the Environmental Level
Abstract
:1. Introduction
2. Results
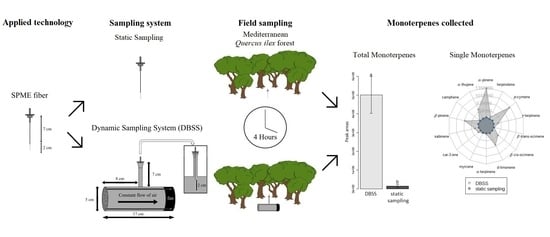
2.1. BVOC Identification and Qualitative Analysis
2.2. Semi-Quantitative Analysis of Individual and Total MTs
3. Discussion
4. Materials and Methods
4.1. Theoretical Background for SPME Sampling in Field Conditions
4.2. Instrumental Setup
4.3. GC-MS Analysis
4.4. Test of DBSS in Field Conditions
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loreto, F.; Schnitzler, J.-P. Abiotic Stresses and Induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Loreto, F.; Fini, A.; Guidi, L.; Brunetti, C.; Velikova, V.; Gori, A.; Ferrini, F. Isoprenoids and Phenylpropanoids Are Part of the Antioxidant Defense Orchestrated Daily by Drought-stressed P Latanus × Acerifolia Plants during Mediterranean Summers. New Phytol. 2015, 207, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Himanen, S.J.; Yuan, J.S.; Chen, F.; Stewart, C.N. Ecological Functions of Terpenoids in Changing Climates. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2913–2940. ISBN 978-3-642-22143-9. [Google Scholar]
- Bonn, B.; Magh, R.-K.; Rombach, J.; Kreuzwieser, J. Biogenic Isoprenoid Emissions under Drought Stress: Different Responses for Isoprene and Terpenes. Biogeosciences 2019, 16, 4627–4645. [Google Scholar] [CrossRef] [Green Version]
- Bertin, N.; Staudt, M. Effect of Water Stress on Monoterpene Emissions from Young Potted Holm Oak (Quercus Ilex L.) Trees. Oecologia 1996, 107, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ormeño, E.; Fernandez, C.; Mévy, J.-P. Plant Coexistence Alters Terpene Emission and Content of Mediterranean Species. Phytochemistry 2007, 68, 840–852. [Google Scholar] [CrossRef]
- Loreto, F.; Pollastri, S.; Fineschi, S.; Velikova, V. Volatile Isoprenoids and Their Importance for Protection against Environmental Constraints in the Mediterranean Area. Environ. Exp. Bot. 2014, 103, 99–106. [Google Scholar] [CrossRef]
- Bach, A.; Yáñez-Serrano, A.M.; Llusià, J.; Filella, I.; Maneja, R.; Penuelas, J. Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. IJERPH 2020, 17, 4391. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Tattini, M.; Loreto, F. Plants in Mediterranean Areas: “Living in the Sun”. Environ. Exp. Bot. 2014, 103, 1–2. [Google Scholar] [CrossRef]
- Steinbrecher, R.; Smiatek, G.; Köble, R.; Seufert, G.; Theloke, J.; Hauff, K.; Ciccioli, P.; Vautard, R.; Curci, G. Intra- and Inter-Annual Variability of VOC Emissions from Natural and Semi-Natural Vegetation in Europe and Neighbouring Countries. Atmos. Environ. 2009, 43, 1380–1391. [Google Scholar] [CrossRef]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature Version 2.1 (MEGAN2.1): An Extended and Updated Framework for Modeling Biogenic Emissions. Geosci. Model. Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef] [Green Version]
- Ghirardo, A.; Xie, J.; Zheng, X.; Wang, Y.; Grote, R.; Block, K.; Wildt, J.; Mentel, T.; Kiendler-Scharr, A.; Hallquist, M.; et al. Urban Stress-Induced Biogenic VOC Emissions and SOA-Forming Potentials in Beijing. Atmos. Chem. Phys. 2016, 16, 2901–2920. [Google Scholar] [CrossRef] [Green Version]
- Griffin, R.J.; Cocker, D.R.; Flagan, R.C.; Seinfeld, J.H. Organic Aerosol Formation from the Oxidation of Biogenic Hydrocarbons. J. Geophys. Res. 1999, 104, 3555–3567. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.H.; Galbally, I.E. Know and Unexplored Organic Constituents in the Earth’s Atmosphere. Environ. Sci. Technol. 2007, 41, 1514–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eerdekens, G.; Yassaa, N.; Sinha, V.; Aalto, P.P.; Aufmhoff, H.; Arnold, F.; Fiedler, V.; Kulmala, M.; Williams, J. VOC Measurements within a Boreal Forest during Spring 2005: On the Occurrence of Elevated Monoterpene Concentrations during Night Time Intense Particle Concentration Events. Atmos. Chem. Phys. 2009, 9, 8331–8350. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.H.; Koven, C.D.; Heald, C.L.; Fung, I.Y. Biogenic Carbon and Anthropogenic Pollutants Combine to Form a Cooling Haze over the Southeastern United States. Proc. Natl. Acad. Sci. USA 2009, 106, 8835–8840. [Google Scholar] [CrossRef] [Green Version]
- Slowik, J.G.; Stroud, C.; Bottenheim, J.W.; Brickell, P.C.; Martin, R.V.; Moran, M.D.; Shantz, N.C.; Sjostedt, S.J.; van Donkelaar, A.; Vlasenko, A.; et al. Characterization of a Large Biogenic Secondary Organic Aerosol Event from Eastern Canadian Forests. Atmos. Chem. Phys. 2010, 10, 2825–2845. [Google Scholar] [CrossRef] [Green Version]
- Arneth, A.; Harrison, S.P.; Zaehle, S.; Tsigaridis, K.; Menon, S.; Bartlein, P.J.; Feichter, J.; Korhola, A.; Kulmala, M.; O’Donnell, D.; et al. Terrestrial Biogeochemical Feedbacks in the Climate System. Nat. Geosci. 2010, 3, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Fares, S.; Schnitzhofer, R.; Jiang, X.; Guenther, A.; Hansel, A.; Loreto, F. Observations of Diurnal to Weekly Variations of Monoterpene-Dominated Fluxes of Volatile Organic Compounds from Mediterranean Forests: Implications for Regional Modeling. Environ. Sci. Technol. 2013, 47, 11073–11082. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of Phytoncide from Trees on Human Natural Killer Cell Function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Li, Q. Effect of Forest Bathing Trips on Human Immune Function. Environ. Health Prev. Med. 2010, 15, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Yan, Q.; Pan, Y.; Gu, X.; Liu, Y. Medical Empirical Research on Forest Bathing (Shinrin-Yoku): A Systematic Review. Environ. Health Prev. Med. 2019, 24, 70. [Google Scholar] [CrossRef] [Green Version]
- Tsunetsugu, Y.; Park, B.-J.; Miyazaki, Y. Trends in Research Related to “Shinrin-Yoku” (Taking in the Forest Atmosphere or Forest Bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Pasquini, D.; Detti, C.; Ferrini, F.; Brunetti, C.; Gori, A. Polyphenols and Terpenes in Mediterranean Plants: An Overview of Their Roles and Possible Applications. Italus Hortus 2021, 28, 3. [Google Scholar] [CrossRef]
- Lan, H.; Hartonen, K.; Riekkola, M.-L. Miniaturised Air Sampling Techniques for Analysis of Volatile Organic Compounds in Air. TrAC Trends Anal. Chem. 2020, 126, 115873. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.-P. Practical Approaches to Plant Volatile Analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef]
- Belardi, R.P.; Pawliszyn, J.B. The Application of Chemically Modified Fused Silica Fibers in the Extraction of Organics from Water Matrix Samples and Their Rapid Transfer to Capillary Columns. Water Qual. Res. J. 1989, 24, 179–191. [Google Scholar] [CrossRef]
- Lord, H.; Pawliszyn, J. Evolution of Solid-Phase Microextraction Technology. J. Chromatogr. A 2000, 885, 153–193. [Google Scholar] [CrossRef]
- Shirey, R.E. Chapter 4—SPME Commercial Devices and Fibre Coating. In Handbook of Solid Phase Microextraction; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Cagliero, C.; Mastellone, G.; Marengo, A.; Bicchi, C.; Sgorbini, B.; Rubiolo, P. Analytical Strategies for In-Vivo Evaluation of Plant Volatile Emissions—A Review. Anal. Chim. Acta 2021, 1147, 240–258. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Kuhn, U.; Harley, P.C.; Staudt, M.; Arneth, A.; Cescatti, A.; Ciccioli, P.; Copolovici, L.; Geron, C.; Guenther, A.; et al. Estimations of Isoprenoid Emission Capacity from Enclosure Studies: Measurements, Data Processing, Quality and Standardized Measurement Protocols. Biogeosciences 2011, 8, 2209–2246. [Google Scholar] [CrossRef] [Green Version]
- Ortega, J.; Helmig, D. Approaches for Quantifying Reactive and Low-Volatility Biogenic Organic Compound Emissions by Vegetation Enclosure Techniques—Part A. Chemosphere 2008, 72, 343–364. [Google Scholar] [CrossRef] [PubMed]
- Asl-Hariri, S.; Gomez-Ríos, G.A.; Gionfriddo, E.; Dawes, P.; Pawliszyn, J. Development of Needle Trap Technology for On-Site Determinations: Active and Passive Sampling. Anal. Chem. 2014, 86, 5889–5897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugheri, S.; Massi, D.; Mucci, N.; Marrubini, G.; Cappelli, G.; Speltini, A.; Bonferoni, M.C.; Arcangeli, G. Exposure to Airborne Formaldehyde: Sampling and Analytical Methods—A Review. Trends Environ. Anal. Chem. 2021, 29, e00116. [Google Scholar] [CrossRef]
- Barreira, L.M.F.; Parshintsev, J.; Kärkkäinen, N.; Hartonen, K.; Jussila, M.; Kajos, M.; Kulmala, M.; Riekkola, M.-L. Field Measurements of Biogenic Volatile Organic Compounds in the Atmosphere by Dynamic Solid-Phase Microextraction and Portable Gas Chromatography-Mass Spectrometry. Atmos. Environ. 2015, 115, 214–222. [Google Scholar] [CrossRef]
- Ruiz-Jimenez, J.; Zanca, N.; Lan, H.; Jussila, M.; Hartonen, K.; Riekkola, M.-L. Aerial Drone as a Carrier for Miniaturized Air Sampling Systems. J. Chromatogr. A 2019, 1597, 202–208. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, G. Chapter 1—Introduction of Solid-Phase Microextraction. In Solid Phase Microextraction: Recent Developments and Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Noe, S.M.; Hüve, K.; Niinemets, Ü.; Copolovici, L. Seasonal Variation in Vertical Volatile Compounds Air Concentrations within a Remote Hemiboreal Mixed Forest. Atmos. Chem. Phys. 2012, 12, 3909–3926. [Google Scholar] [CrossRef] [Green Version]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Roces-Díaz, J.V.; Vayreda, J.; De Cáceres, M.; García-Valdés, R.; Banqué-Casanovas, M.; Morán-Ordóñez, A.; Brotons, L.; de-Miguel, S.; Martínez-Vilalta, J. Temporal Changes in Mediterranean Forest Ecosystem Services Are Driven by Stand Development, Rather than by Climate-Related Disturbances. For. Ecol. Manag. 2021, 480, 118623. [Google Scholar] [CrossRef]
- Tuffery, L.; Davi, H.; López-García, N.; Rigolot, E.; Jean, F.; Stenger, A.; Lefèvre, F. Adaptive Measures for Mountain Mediterranean Forest Ecosystem Services under Climate and Land Cover Change in the Mont-Ventoux Regional Nature Park, France. Reg. Environ. Chang. 2021, 21, 12. [Google Scholar] [CrossRef]
- Morán-Ordóñez, A.; Ameztegui, A.; De Cáceres, M.; de-Miguel, S.; Lefèvre, F.; Brotons, L.; Coll, L. Future Trade-Offs and Synergies among Ecosystem Services in Mediterranean Forests under Global Change Scenarios. Ecosyst. Serv. 2020, 45, 101174. [Google Scholar] [CrossRef]
- Gentilesca, T.; Camarero, J.; Colangelo, M.; Nolè, A.; Ripullone, F. Drought-Induced Oak Decline in the Western Mediterranean Region: An Overview on Current Evidences, Mechanisms and Management Options to Improve Forest Resilience. iForest 2017, 10, 796–806. [Google Scholar] [CrossRef] [Green Version]
- Yassaa, N.; Custer, T.; Song, W.; Pech, F.; Kesselmeier, J.; Williams, J. Quantitative and Enantioselective Analysis of Monoterpenes from Plant Chambers and in Ambient Air Using SPME. Atmos. Meas. Tech. 2010, 3, 1615–1627. [Google Scholar] [CrossRef] [Green Version]
- Staudt, M.; Joffre, R.; Rambal, S.; Kesselmeier, J. Effect of Elevated CO2 on Monoterpene Emission of Young Quercus Ilex Trees and Its Relation to Structural and Ecophysiological Parameters. Tree Physiol. 2001, 21, 437–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabillo, D. Diurnal and Seasonal Variation of Monoterpene Emission Rates for Two Typical Mediterranean Species (Pinus Pinea and Quercus Ilex) from FIeld MeasurementsFrelationship with Temperature and PAR. Atmos. Environ. 2001, 35, 4419–4431. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. Seasonal Emission of Monoterpenes by the Mediterranean Tree Quercus Ilex in Field Conditions: Relations with Photosynthetic Rates, Temperature and Volatility. Physiol. Plant. 1999, 105, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Loreto, F.; Forster, A.; Durr, M.; Csiky, O.; Seufert, G. On the Monoterpene Emission under Heat Stress and on the Increased Thermotolerance of Leaves of Quercus Ilex L. Fumigated with Selected Monoterpenes. Plant Cell Environ. 1998, 21, 101–107. [Google Scholar] [CrossRef]
- Bsaibes, S.; Piel, F.; Gros, V.; Truong, F.; Lafouge, F.; Ciuraru, R.; Buysse, P.; Kammer, J.; Loubet, B.; Staudt, M. Monoterpene Chemical Speciation with High Time Resolution Using a FastGC/PTR-MS: Results from the COV3ER Experiment on Quercus Ilex. Atmosphere 2020, 11, 690. [Google Scholar] [CrossRef]
- Asensio, D.; Peñuelas, J.; Prieto, P.; Estiarte, M.; Filella, I.; Llusià, J. Interannual and Seasonal Changes in the Soil Exchange Rates of Monoterpenes and Other VOCs in a Mediterranean Shrubland. Eur. J. Soil Sci. 2008, 59, 878–891. [Google Scholar] [CrossRef]
- Viros, J.; Fernandez, C.; Wortham, H.; Gavinet, J.; Lecareux, C.; Ormeño, E. Litter of Mediterranean Species as a Source of Volatile Organic Compounds. Atmos. Environ. 2020, 242, 117815. [Google Scholar] [CrossRef]
- Adam, M.; Juklová, M.; Bajer, T.; Eisner, A.; Ventura, K. Comparison of Three Different Solid-Phase Microextraction Fibres for Analysis of Essential Oils in Yacon (Smallanthus Sonchifolius) Leaves. J. Chromatogr. A 2005, 1084, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Quan, W.; Li, C.; Xiang, Z. Analysis of Volatile Terpenoid Compounds in Rhododendron Species by Multidimensional Gas Chromatography with Quadrupole Time-of-Flight Mass Spectrometry. Microchem. J. 2019, 149, 104064. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Changes in Terpene Content and Emission in Potted Mediterranean Woody Plants under Severe Drought. Can. J. Bot. 1998, 76, 1366–1373. [Google Scholar] [CrossRef]
- Li, L.Y.; Chen, Y.; Xie, S.D. Spatio-Temporal Variation of Biogenic Volatile Organic Compounds Emissions in China. Environ. Pollut. 2013, 182, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Guenther, A.; Turnipseed, A.; Duhl, T.; Greenberg, J. Seasonal and Interannual Variations in Whole-Ecosystem BVOC Emissions from a Subtropical Plantation in China. Atmos. Environ. 2017, 161, 176–190. [Google Scholar] [CrossRef]
- Penuelas, J.; Llusia, J.; Asensio, D.; Munne-Bosch, S. Linking Isoprene with Plant Thermotolerance, Antioxidants and Monoterpene Emissions. Plant Cell Environ. 2005, 28, 278–286. [Google Scholar] [CrossRef]
- Martos, P.A.; Pawliszyn, J. Calibration of Solid Phase Microextraction for Air Analyses Based on Physical Chemical Properties of the Coating. Anal. Chem. 1997, 69, 206–215. [Google Scholar] [CrossRef]
- Strong, C.; Fuentes, J.D.; Baldocchi, D. Reactive Hydrocarbon FLux Footprints during Canopy Senescence. Agric. For. Meteorol. 2004, 27, 159–173. [Google Scholar] [CrossRef]
- Fuentes, J.D.; Wang, D.; Bowling, D.R.; Potosnak, M.; Monson, R.K.; Goliff, W.S.; Stockwell, W.R. Biogenic Hydrocarbon Chemistry within and Above a Mixed Deciduous Forest. J. Atmos. Chem. 2007, 56, 165–185. [Google Scholar] [CrossRef]
- Fick, J.; Pommer, L.; Andersson, B.; Nilsson, C. Ozone Removal in the Sampling of Parts per Billion Levels of Terpenoid Compounds: An Evaluation of Different Scrubber Materials. Environ. Sci. Technol. 2001, 35, 1458–1462. [Google Scholar] [CrossRef]
- Mermet, K.; Perraudin, E.; Dusanter, S.; Sauvage, S.; Léonardis, T.; Flaud, P.-M.; Bsaibes, S.; Kammer, J.; Michoud, V.; Gratien, A.; et al. Atmospheric Reactivity of Biogenic Volatile Organic Compounds in a Maritime Pine Forest during the LANDEX Episode 1 Field Campaign. Sci. Total. Environ. 2021, 756, 144129. [Google Scholar] [CrossRef]
- Heil, M. Herbivore-Induced Plant Volatiles: Targets, Perception and Unanswered Questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Llusià, J.; Penuelas, J. Seasonal Patterns of Terpene Content and Emission from Seven Mediterranean Woody Species in Field Conditions. Am. J. Bot. 2000, 87, 8. [Google Scholar] [CrossRef] [Green Version]
- Llusia, J.; Penuelas, J.; Asensio, D.; Munne-Bosch, S. Airborne Limonene Confers Limited Thermotolerance to Quercus Ilex. Physiol. Plant 2005, 123, 40–48. [Google Scholar] [CrossRef]
- Blanch, J.-S.; Peñuelas, J.; Sardans, J.; Llusià, J. Drought, Warming and Soil Fertilization Effects on Leaf Volatile Terpene Concentrations in Pinus Halepensis and Quercus Ilex. Acta Physiol. Plant 2009, 31, 207–218. [Google Scholar] [CrossRef]
- Bae, G.-S.; Park, K.-C.; Choi, S.B.; Jo, I.-J.; Choi, M.-O.; Hong, S.-H.; Song, K.; Song, H.-J.; Park, S.-J. Protective Effects of Alpha-Pinene in Mice with Cerulein-Induced Acute Pancreatitis. Life Sci. 2012, 91, 866–871. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.W.; Lee, B.S.; Kim, S.H.; et al. Apha-Pinene Exhibits Anti-Inflammatory Activity through the Suppression of MAPKs and the NF-KB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Cândido, A.G.F.; Wong, D.V.T.; et al. α-Phellandrene, a Cyclic Monoterpene, Attenuates Inflammatory Response through Neutrophil Migration Inhibition and Mast Cell Degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.L.; Simas, D.L.R.; Pinheiro, M.M.G.; Moreno, D.S.A.; Alviano, C.S.; da Silva, A.J.R.; Dias Fernandes, P. Anti-Inflammatory Properties and Chemical Characterization of the Essential Oils of Four Citrus Species. PLoS ONE 2016, 11, e0153643. [Google Scholar] [CrossRef] [Green Version]
- Games, E.; Guerreiro, M.; Santana, F.; Pinheiro, N.; de Oliveira, E.; Lopes, F.; Olivo, C.; Tibério, I.; Martins, M.; Lago, J.; et al. Structurally Related Monoterpenes P-Cymene, Carvacrol and Thymol Isolated from Essential Oil from Leaves of Lippia Sidoides Cham. (Verbenaceae) Protect Mice against Elastase-Induced Emphysema. Molecules 2016, 21, 1390. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Lee, C.J. Sleep-Enhancing Effects of Phytoncide Via Behavioral, Electrophysiological, and Molecular Modeling Approaches. Exp. Neurobiol. 2020, 29, 120–129. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The Antioxidant Effect of β-Caryophyllene Protects Rat Liver from Carbon Tetrachloride-Induced Fibrosis by Inhibiting Hepatic Stellate Cell Activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene Ameliorates the Alzheimer-Like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: Chennai, Thailand; Oxford, UK, 2012; ISBN 978-0-12-416017-0. [Google Scholar]
- Ai, J. Solid Phase Microextraction for Quantitative Analysis in Nonequilibrium Situations. Anal. Chem. 1997, 69, 1230–1236. [Google Scholar] [CrossRef]
- Ai, J. Headspace Solid Phase Microextraction. Dynamics and Quantitative Analysis before Reaching a Partition Equilibrium. Anal. Chem. 1997, 69, 3260–3266. [Google Scholar] [CrossRef]
- Goodner, K.L. Practical Retention Index Models of OV-101, DB-1, DB-5, and DB-Wax for FLavor and Fragrance Compounds$. LWT-Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- Vezzola, L.C.; Michelozzi, M.; Calamai, L.; Gonthier, P.; Giordano, L.; Cherubini, P.; Pelfini, M. Tree-Ring Volatile Terpenes Show Potential to Indicate Fungal Infection in Asymptomatic Mature Norway Spruce Trees in the Alps. For. Int. J. For. Res. 2019, 92, 149–156. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Oxford Journals 1965, 52, 591–611. [Google Scholar]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The Impact of Levene’s Test of Equality of Variances on Statistical Theory and Practice. Statist. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Nordstokke, D.W.; Zumbo, B.D. A New Nonparametric Levene Test for Equal Variances. Psicologica 2010, 31, 401–430. [Google Scholar]






| n. Peak | Compound Identified | RT | Presence | ||
|---|---|---|---|---|---|
| (min) | SP 1 (dynamic/static) | SP 2 (dynamic/static) | SP 3 (dynamic/static) | ||
| 1 | α-pinene | 10.07 ± 0.02 | y/y | y/y | y/y |
| 2 | α-thujene | 10.15 ± 0.02 | y/y | y/y | y/y |
| 3 | camphene | 11.35 ± 0.05 | y/y | y/y | y/y |
| 4 | β-pinene | 12.61 ± 0.04 | y/y | y/y | y/y |
| 5 | sabinene | 12.76 ± 0.05 | y/y | y/y | y/y |
| 6 | car-3-ene | 12.97 ± 0.03 | y/y | y/y | y/n |
| 7 | β-phellandrene | 13.10 ± 0.03 | n/n | y/y | n/n |
| 8 | myrcene | 14.12 ± 0.02 | n/n | y/y | y/n |
| 9 | α-phellandrene | 14.35 ± 0.02 | n/n | y/y | n/n |
| 10 | α-terpinene | 14.83 ± 0.04 | n/n | y/y | y/n |
| 11 | d-limonene | 15.39 ± 0.02 | y/y | y/y | y/y |
| 12 | 1,8-cineole | 15.68 ± 0.01 | y/y | n/n | n/n |
| 13 | β-cis-ocimene | 15.72 ± 0.02 | n/n | y/y | y/y |
| 14 | β-trans-ocimene | 13.99 ± 0.02 | n/n | n/n | y/n |
| 15 | γ-terpinene | 16.80 ± 0.01 | n/n | y/y | y/n |
| 16 | p-cymene | 17.62 ± 0.02 | y/y | y/y | y/y |
| 17 | terpinolene | 17.95 ± 0.01 | n/n | y/y | y/n |
| Sampling Point | Sampling Day | Temperature | Humidity | Wind | Sampling Time | Plants | Site |
|---|---|---|---|---|---|---|---|
| N | dd/mm/yyyy | °C | % | speed (km/h), direction | |||
| 1 | 6/6/2019 | 24 °C | 45% | 9–14 km/h, W/SW | 12 pm 4pm | Q. ilex in pot | Sesto Fiorentino (FI) |
| 2 | 20/06/2019 | 26.5 °C | 45% | 10 km/h, W/SW | 12 pm 4 pm | Q. ilex forest | Maremma Regional Park (GR) |
| 3 | 1/10/2019 | 20 °C | 60% | 8km/h, S | 12 pm 4 pm | Q. ilex forest | Maremma Regional Park (GR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquini, D.; Gori, A.; Ferrini, F.; Brunetti, C. An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus ilex at the Environmental Level. Metabolites 2021, 11, 388. https://doi.org/10.3390/metabo11060388
Pasquini D, Gori A, Ferrini F, Brunetti C. An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus ilex at the Environmental Level. Metabolites. 2021; 11(6):388. https://doi.org/10.3390/metabo11060388
Chicago/Turabian StylePasquini, Dalila, Antonella Gori, Francesco Ferrini, and Cecilia Brunetti. 2021. "An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus ilex at the Environmental Level" Metabolites 11, no. 6: 388. https://doi.org/10.3390/metabo11060388
APA StylePasquini, D., Gori, A., Ferrini, F., & Brunetti, C. (2021). An Improvement of SPME-Based Sampling Technique to Collect Volatile Organic Compounds from Quercus ilex at the Environmental Level. Metabolites, 11(6), 388. https://doi.org/10.3390/metabo11060388