Ectopic Leptin Production by Intraocular Pancreatic Islet Organoids Ameliorates the Metabolic Phenotype of ob/ob Mice
Abstract
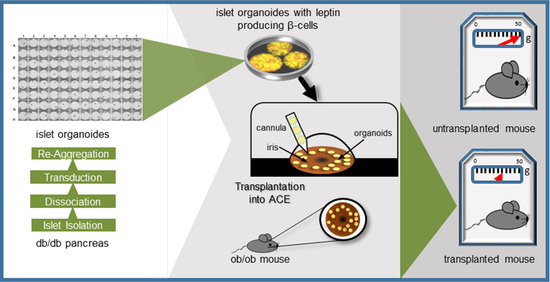
:1. Introduction
2. Results
2.1. Generation of a Tet-OFF-Based Beta-Cell Specific Adenoviral Expression Construct for Mouse Leptin and Its In Vitro Assessment
2.2. Ectopic Leptin Production by Islet Organoid Grafts in the ACE Ameliorates the Metabolic Phenotype of ob/ob Mice
2.3. Doxycycline Treatment Stops Ectopic Leptin Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Leptin Treatment of ob/ob Mice
4.3. Expression Vector Construction
4.4. Isolation of Pancreatic Islets
4.5. Islet Organoid Production
4.6. In Vitro Imaging of Intraocular Islet Organoids
4.7. Leptin Secretion from Islet Organoids In Vitro
4.8. Transplantation of Islet Organoids into the ACE
4.9. In Vivo Imaging of Intraocular Islet Organoid Grafts
4.10. Doxycycline Treatment of Animals
4.11. Body Weight and Fasting and Non-Fasting Blood Glucose
4.12. Intraperitoneal Glucose Tolerance Test (ipGTT)
4.13. Plasma and Aqueous Humor Samples
4.14. Insulin and C-Peptide Measurements
4.15. Leptin Measurements
4.16. Tissue Extraction and Sectioning
4.17. Immunofluorescence in Eye Sections
4.18. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A


References
- Speier, S.; Nyqvist, D.; Cabrera, O.; Yu, J.; Molano, R.D.; Pileggi, A.; Moede, T.; Köhler, M.; Wilbertz, J.; Leibiger, B.; et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat. Med. 2008, 14, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leibiger, I.B.; Berggren, P.-O. Intraocular in vivo imaging of pancreatic islet cell physiology/pathology. Mol. Metab. 2017, 6, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Van Krieken, P.P.; Voznesenskaya, A.; Dicker, A.; Xiong, Y.; Park, J.H.; Lee, J.I.; Ilegems, E.; Berggren, P.-O. Translational assessment of a genetic engineering methodology to improve islet function for transplantation. EBioMedicine 2019, 45, 529–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir-Coll, J.; Moede, T.; Paschen, M.; Neelakandhan, A.; Valladolid-Acebes, I.; Leibiger, B.; Biernath, A.; Ämmälä, C.; Leibiger, I.; Yesildag, B.; et al. Human Islet Microtissues as an In Vitro and an In Vivo Model System for Diabetes. Int. J. Mol. Sci. 2021, 22, 1813. [Google Scholar] [CrossRef] [PubMed]
- Lindström, P. The Physiology of Obese-Hyperglycemic Mice [ob/obMice]. Sci. World J. 2007, 7, 666–685. [Google Scholar] [CrossRef] [Green Version]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Halaas, J.L.; Boozer, C.; Blair-West, J.; Fidahusein, N.; Denton, D.; Friedman, J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl. Acad. Sci. USA 1997, 94, 8878–8883. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.B.S.; Zhou, J.; Redmann, S.M.; Smagin, G.N.; Smith, S.R.; Rodgers, E.; Zachwieja, J.J. A Leptin Dose-Response Study in Obese (ob/ob) and Lean (+/?) Mice. Endocrinology 1998, 139, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Gu, M.C.; Zhao, J.Z.; Holder, D.J.; Rogers, I.T.; Pouch, W.J.; Motzel, S.L.; Klein, H.J.; Gupta, S.K.; Liang, X.; et al. Leptin gene therapy and daily protein administration: A comparative study in the ob/ob mouse. Gene Ther. 1998, 5, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, H.; Ge, Y.-L.; Minter, R.; Prima, V.; Moldawer, L.; Muzyczka, N.; Zolotukhin, S.; Kalra, P.; Kalra, S. Long-term differential modulation of genes encoding orexigenic and anorexigenic peptides by leptin delivered by rAAV vector in ob/ob mice. Regul. Pept. 2000, 92, 97–105. [Google Scholar] [CrossRef]
- Oosman, S.N.; Lam, A.W.; Harb, G.; Unniappan, S.; Lam, N.T.; Webber, T.; Bruch, D.; Zhang, Q.-X.; Korbutt, G.S.; Kieffer, T.J. Treatment of Obesity and Diabetes in Mice by Transplant of Gut Cells Engineered to Produce Leptin. Mol. Ther. 2008, 16, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Larcher, F.; Del Rio, M.; Serrano, F.; Segovia, J.C.; Ramirez, A.; Meana, A.; Page, A.; Abad, J.L.; González, M.A.; Bueren, J.; et al. A cutaneous gene therapy approach to human leptin deficiencies: Correction of the murine ob/ob phenotype using leptin-targeted keratinocyte grafts. FASEB J. 2001, 15, 1529–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, A.M.; Neumann, U.H.; Glavas, M.M.; Kieffer, T.J. The glucoregulatory actions of leptin. Mol. Metab. 2017, 6, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Ilegems, E.; Dicker, A.; Speier, S.; Sharma, A.; Bahow, A.; Edlund, P.K.; Leibiger, I.B.; Berggren, P.-O. Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proc. Natl. Acad. Sci. USA 2013, 110, 20581–20586. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.N.; Wang, Z.L.; Wang, R.M.; Hurley, J.D.; Smith, D.M.; Ghatei, M.A.; Withers, D.J.; Gardiner, J.V.; Bailey, C.J.; Bloom, S.R. Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J. Clin. Investig. 1997, 100, 2729–2736. [Google Scholar] [CrossRef]
- Åvall, K.; Ali, Y.; Leibiger, I.B.; Leibiger, B.; Moede, T.; Paschen, M.; Dicker, A.; Daré, E.; Köhler, M.; Ilegems, E.; et al. Apolipoprotein CIII links islet insulin resistance to β-cell failure in diabetes. Proc. Natl. Acad. Sci. USA 2015, 112, E2611–E2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiSilvestro, D.J.; Melgar-Bermudez, E.; Yasmeen, R.; Fadda, P.; Lee, L.J.; Kalyanasundaram, A.; Gilor, C.L.; Ziouzenkova, O. Leptin Production by Encapsulated Adipocytes Increases Brown Fat, Decreases Resistin, and Improves Glucose Intolerance in Obese Mice. PLoS ONE 2016, 11, e0153198. [Google Scholar] [CrossRef]
- Muzzin, P.; Eisensmith, R.C.; Copeland, K.C.; Woo, S.L.C. Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proc. Natl. Acad. Sci. USA 1996, 93, 14804–14808. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.; Miller, K.; Jo, J.; Kilimnik, G.; Wojcik, P.; Hara, M. Islet architecture: A comparative study. ISLETS 2009, 1, 129–136. [Google Scholar] [CrossRef]
- Yang, Y.; Nunes, F.A.; Berencsi, K.; Furth, E.E.; Gonczol, E.; Wilson, J. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 1994, 91, 4407–4411. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, S.K.; Black, H.B.; Goldwasser, E.; Leiden, J.M. Immune responses to transgene–encoded proteins limit the stability of gene expression after injection of replication–defective adenovirus vectors. Nat. Med. 1996, 2, 545–550. [Google Scholar] [CrossRef]
- Paschen, M.; Moede, T.; Valladolid-Acebes, I.; Leibiger, B.; Moruzzi, N.; Jacob, S.; García-Prieto, C.F.; Brismar, K.; Leibiger, I.B.; Berggren, P.-O. Diet-induced β-cell insulin resistance results in reversible loss of functional β-cell mass. FASEB J. 2019, 33, 204–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speier, S.; Nyqvist, D.; Köhler, M.; Caicedo, A.; Leibiger, I.B.; Berggren, P.-O. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat. Protoc. 2008, 3, 1278–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leibiger, B.; Moede, T.; Valladolid-Acebes, I.; Paschen, M.; Visa, M.; Leibiger, I.B.; Berggren, P.-O. Ectopic Leptin Production by Intraocular Pancreatic Islet Organoids Ameliorates the Metabolic Phenotype of ob/ob Mice. Metabolites 2021, 11, 387. https://doi.org/10.3390/metabo11060387
Leibiger B, Moede T, Valladolid-Acebes I, Paschen M, Visa M, Leibiger IB, Berggren P-O. Ectopic Leptin Production by Intraocular Pancreatic Islet Organoids Ameliorates the Metabolic Phenotype of ob/ob Mice. Metabolites. 2021; 11(6):387. https://doi.org/10.3390/metabo11060387
Chicago/Turabian StyleLeibiger, Barbara, Tilo Moede, Ismael Valladolid-Acebes, Meike Paschen, Montse Visa, Ingo B. Leibiger, and Per-Olof Berggren. 2021. "Ectopic Leptin Production by Intraocular Pancreatic Islet Organoids Ameliorates the Metabolic Phenotype of ob/ob Mice" Metabolites 11, no. 6: 387. https://doi.org/10.3390/metabo11060387
APA StyleLeibiger, B., Moede, T., Valladolid-Acebes, I., Paschen, M., Visa, M., Leibiger, I. B., & Berggren, P. -O. (2021). Ectopic Leptin Production by Intraocular Pancreatic Islet Organoids Ameliorates the Metabolic Phenotype of ob/ob Mice. Metabolites, 11(6), 387. https://doi.org/10.3390/metabo11060387