Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population
Abstract
:1. Introduction
2. Results
2.1. Population Demographic and Clinical Characteristics
2.2. Serum Metabolite Profile According to Sex
2.3. Serum Metabolite Profile According to VAI
2.4. Metabolic Profile According to Sex-Dependent VAI
2.5. Metabolite Set Enrichment Analysis
2.6. Metabolic Profile According to BMD Status
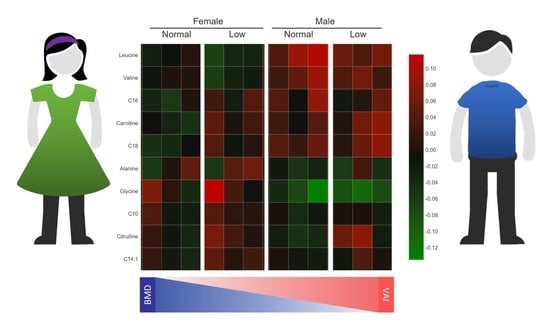
2.7. Metabolic Profile According to Sex-Dependent VAI and BMD Status
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. BMD Measurements
4.3. Other Measurements
4.4. Metabolomics Analysis
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Kumar, P.; Mahalingam, K. Molecular genetics of human obesity: A comprehensive review. C. R. Biol. 2017, 340, 87–108. [Google Scholar] [CrossRef]
- Yuan, Y.; Mu, J.-J.; Chu, C.; Zheng, W.-L.; Wang, Y.; Hu, J.-W.; Ma, Q.; Yan, Y.; Liao, Y.-Y.; Chen, C. Effect of metabolically healthy obesity on the development of arterial stiffness: A prospective cohort study. Nutr. Metab. 2020, 17, 50. [Google Scholar] [CrossRef]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. Eat. Weight Disord. 2018, 23, 293–302. [Google Scholar] [CrossRef]
- Zamani, M.; Zamani, V.; Heidari, B.; Parsian, H.; Esmaeilnejad-Ganji, S.M. Prevalence of osteoporosis with the World Health Organization diagnostic criteria in the Eastern Mediterranean Region: A systematic review and meta-analysis. Arch Osteoporos. 2018, 13, 129. [Google Scholar] [CrossRef]
- Instituto Nacional de Salud Pública (INSP) Encuesta Nacional de Salud y Nutrición 2018 (ENSANUT-2018-2019). Available online: https://ensanut.insp.mx/encuestas/ensanut2018/doctos/informes/ensanut_2018_presentacion_resultados.pdf (accessed on 17 May 2021).
- National Osteoporosis Foundation. Annual Report. Available online: https://cdn.nof.org/wp-content/uploads/Annual-Report-2019-2.pdf (accessed on 17 May 2021).
- Drake, M.T.; Clarke, B.L.; Lewiecki, E.M. The Pathophysiology and Treatment of Osteoporosis. Clin. Ther. 2015, 37, 1837–1850. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Rand, M.S.; Eiken, P.A.; Jørgensen, N.R. Monitoring anti-osteoporotic therapy: Bone mineral density or markers of bone turnover. Ugeskr. Laeger 2018, 180. [Google Scholar]
- Liu, L.; Yang, X.-L.; Zhang, H.; Zhang, Z.-J.; Wei, X.-T.; Feng, G.-J.; Liu, J.; Peng, H.-P.; Hai, R.; Shen, H.; et al. Two novel pleiotropic loci associated with osteoporosis and abdominal obesity. Hum. Genet. 2020, 139, 1023–1035. [Google Scholar] [CrossRef]
- Fall, T.; Ingelsson, E. Genome-wide association studies of obesity and metabolic syndrome. Mol. Cell. Endocrinol. 2014, 382, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-J.; Liu, Y.-J.; Liu, P.-Y.; Hamilton, J.; Recker, R.R.; Deng, H.-W. Relationship of obesity with osteoporosis. J Clin. Endocrinol. Metab. 2007, 92, 1640–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kushwaha, P.; Wolfgang, M.J.; Riddle, R.C. Fatty acid metabolism by the osteoblast. Bone 2018, 115, 8–14. [Google Scholar] [CrossRef]
- Cohen, A.; Dempster, D.W.; Recker, R.R.; Lappe, J.M.; Zhou, H.; Zwahlen, A.; Müller, R.; Zhao, B.; Guo, X.; Lang, T.; et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: A transiliac bone biopsy study. J. Clin. Endocrinol. Metab. 2013, 98, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Barreira, T.V.; Harrington, D.M.; Staiano, A.E.; Heymsfield, S.B.; Gimble, J.M. Relationship between abdominal fat and bone mineral density in white and African American adults. Bone 2012, 50, 576–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pou, K.M.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; Maurovich-Horvat, P.; Larson, M.G.; Keaney, J.F.J.; Meigs, J.B.; Lipinska, I.; Kathiresan, S.; et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: The Framingham Heart Study. Circulation 2007, 116, 1234–1241. [Google Scholar] [CrossRef] [Green Version]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, D.; Kruger, M.; Wolber, F.M.; Roy, N.C.; Totman, J.J.; Henry, C.J.; Cameron-Smith, D.; Fraser, K. Association of Plasma Lipids and Polar Metabolites with Low Bone Mineral Density in Singaporean-Chinese Menopausal Women: A Pilot Study. Int. J. Environ. Res. Public Health 2018, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- De Lorenzo, A.; Soldati, L.; Sarlo, F.; Calvani, M.; Di Lorenzo, N.; Di Renzo, L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016, 22, 681–703. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef] [Green Version]
- Bozorgmanesh, M.; Hadaegh, F.; Azizi, F. Predictive performance of the visceral adiposity index for a visceral adiposity-related risk: Type 2 diabetes. Lipids Health Dis. 2011, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.J.; Ma, F.; Lou, H.P.; Chen, Y. Visceral Adiposity Index Is Associated with Pre-Diabetes and Type 2 Diabetes Mellitus in Chinese Adults Aged 20–50. Ann. Nutr. Metab. 2016, 68, 235–243. [Google Scholar] [CrossRef]
- Wei, J.; Liu, X.; Xue, H.; Wang, Y.; Shi, Z. Comparisons of Visceral Adiposity Index, Body Shape Index, Body Mass Index and Waist Circumference and Their Associations with Diabetes Mellitus in Adults. Nutrients 2019, 11, 1580. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [Green Version]
- Jabłonowska-Lietz, B.; Wrzosek, M.; Włodarczyk, M.; Nowicka, G. New indexes of body fat distribution, visceral adiposity index, body adiposity index, waist-to-height ratio, and metabolic disturbances in the obese. Kardiol. Pol. 2017, 75, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Cornier, M.-A.; Després, J.-P.; Davis, N.; Grossniklaus, D.A.; Klein, S.; Lamarche, B.; Lopez-Jimenez, F.; Rao, G.; St-Onge, M.-P.; Towfighi, A.; et al. Assessing adiposity: A scientific statement from the American Heart Association. Circulation 2011, 124, 1996–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, C.; O’Grada, C.; Ryan, M.; Roche, H.M.; Gibney, M.J.; Gibney, E.R.; Brennan, L. The relationship between BMI and metabolomic profiles: A focus on amino acids. Proc. Nutr. Soc. 2012, 71, 634–638. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yan, D.; Zhao, A.; Hou, X.; Zheng, X.; Chen, P.; Bao, Y.; Jia, W.; Hu, C.; Zhang, Z.-L.; et al. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporos. Int. 2019, 30, 1491–1499. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Li, G.H.; Long, M.T.; Cheung, C.-L.; Vasan, R.S.; Hsu, Y.-H.; Kiel, D.P.; Liu, C.-T. Metabolomics Insights into Osteoporosis through Association with Bone Mineral Density. J. Bone Miner. Res. 2021, 36, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bao, J.; An, G.; Ouyang, G.; Zhang, P.; Wang, C.; Ying, H.; Ouyang, P.; Ma, B.; Zhang, Q. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Mol. Biosyst. 2016. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T.J. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- Connelly, M.A.; Wolak-Dinsmore, J.; Dullaart, R.P.F. Branched Chain Amino Acids Are Associated with Insulin Resistance Independent of Leptin and Adiponectin in Subjects with Varying Degrees of Glucose Tolerance. Metab. Syndr. Relat. Disord. 2017, 15, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, B.B.; Myers, M.G.J. mTOR tells the brain that the body is hungry. Nat. Med. 2006, 12, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. 2019, 34, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.Y.; Kim, O.Y.; Ham, B.M.; Kim, H.-J.; Kwon, D.Y.; Jang, Y.; Lee, J.H. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS). J. Proteome Res. 2010, 9, 4368–4375. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Ma, X.; Zhao, A.; Wang, C.; Zhang, Y.; Nieman, D.; Nicholson, J.K.; Jia, W.; Bao, Y.; Jia, W. The metabolite profiles of the obese population are gender-dependent. J. Proteome Res. 2014, 13, 4062–4073. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [Green Version]
- Mai, M.; Tönjes, A.; Kovacs, P.; Stumvoll, M.; Fiedler, G.M.; Leichtle, A.B. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS ONE 2013, 8, e82459. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Roman, M.A.; Sweetman, L.; Valdez, M.J.; Parks, E.J. Postprandial changes in plasma acylcarnitine concentrations as markers of fatty acid flux in overweight and obesity. Metabolism 2012, 61, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, C.; Chen, L.; Han, X.; Ji, L. Human serum acylcarnitine profiles in different glucose tolerance states. Diabetes Res. Clin. Pract. 2014, 104, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Hoppel, C.L.; Lok, K.H.; Zhao, L.; Wong, S.W.; Minkler, P.E.; Hwang, D.H.; Newman, J.W.; Garvey, W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Dadson, P.; Rebelos, E.; Honka, H.; Juárez-Orozco, L.E.; Kalliokoski, K.K.; Iozzo, P.; Teuho, J.; Salminen, P.; Pihlajamäki, J.; Hannukainen, J.C.; et al. Change in abdominal, but not femoral subcutaneous fat CT-radiodensity is associated with improved metabolic profile after bariatric surgery. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2363–2371. [Google Scholar] [CrossRef]
- Hartstra, A.V.; de Groot, P.F.; Mendes Bastos, D.; Levin, E.; Serlie, M.J.; Soeters, M.R.; Pekmez, C.T.; Dragsted, L.O.; Ackermans, M.T.; Groen, A.K.; et al. Correlation of plasma metabolites with glucose and lipid fluxes in human insulin resistance. Obes. Sci. Pract. 2020, 6, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.P.; Asher, G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017, 25, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Palmnäs, M.S.A.; Kopciuk, K.A.; Shaykhutdinov, R.A.; Robson, P.J.; Mignault, D.; Rabasa-Lhoret, R.; Vogel, H.J.; Csizmadi, I. Serum Metabolomics of Activity Energy Expenditure and its Relation to Metabolic Syndrome and Obesity. Sci. Rep. 2018, 8, 3308. [Google Scholar] [CrossRef] [Green Version]
- Simmons, R.M.; McKnight, S.M.; Edwards, A.K.; Wu, G.; Satterfield, M.C. Obesity increases hepatic glycine dehydrogenase and aminomethyltransferase expression while dietary glycine supplementation reduces white adipose tissue in Zucker diabetic fatty rats. Amino Acids 2020, 52, 1413–1423. [Google Scholar] [CrossRef]
- Sadria, M.; Layton, A.T. Interactions among mTORC, AMPK and SIRT: A computational model for cell energy balance and metabolism. Cell Commun. Signal. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Eshreif, A.; Al Batran, R.; Jamieson, K.L.; Darwesh, A.M.; Gopal, K.; Greenwell, A.A.; Zlobine, I.; Aburasayn, H.; Eaton, F.; Mulvihill, E.E.; et al. l-Citrulline supplementation improves glucose and exercise tolerance in obese male mice. Exp. Physiol. 2020, 105, 270–281. [Google Scholar] [CrossRef]
- Ramírez-Zamora, S.; Méndez-Rodríguez, M.L.; Olguín-Martínez, M.; Sánchez-Sevilla, L.; Quintana-Quintana, M.; García-García, N.; Hernández-Muñoz, R. Increased erythrocytes by-products of arginine catabolism are associated with hyperglycemia and could be involved in the pathogenesis of type 2 diabetes mellitus. PLoS ONE 2013, 8, e66823. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Le Plenier, S.; Noirez, P.; Morio, B.; Bonnefont-Rousselot, D.; Kharchi, C.; Ferry, A.; Neveux, N.; Cynober, L.; Raynaud-Simon, A. Citrulline Supplementation Induces Changes in Body Composition and Limits Age-Related Metabolic Changes in Healthy Male Rats. J. Nutr. 2015, 145, 1429–1437. [Google Scholar] [CrossRef]
- Bouillanne, O.; Melchior, J.-C.; Faure, C.; Paul, M.; Canouï-Poitrine, F.; Boirie, Y.; Chevenne, D.; Forasassi, C.; Guery, E.; Herbaud, S.; et al. Impact of 3-week citrulline supplementation on postprandial protein metabolism in malnourished older patients: The Ciproage randomized controlled trial. Clin. Nutr. 2019, 38, 564–574. [Google Scholar] [CrossRef]
- Kochhar, S.; Jacobs, D.M.; Ramadan, Z.; Berruex, F.; Fuerholz, A.; Fay, L.B. Probing gender-specific metabolism differences in humans by nuclear magnetic resonance-based metabonomics. Anal. Biochem. 2006, 352, 274–281. [Google Scholar] [CrossRef]
- Filho, J.C.; Bergström, J.; Stehle, P.; Fürst, P. Simultaneous measurements of free amino acid patterns of plasma, muscle and erythrocytes in healthy human subjects. Clin. Nutr. 1997, 16, 299–305. [Google Scholar] [CrossRef]
- Kobayashi, R.; Shimomura, Y.; Murakami, T.; Nakai, N.; Fujitsuka, N.; Otsuka, M.; Arakawa, N.; Popov, K.M.; Harris, R.A. Gender difference in regulation of branched-chain amino acid catabolism. Biochem. J. 1997, 327 Pt 2, 449–453. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Gender differences in metabolism, nutrition and supplements. J. Sci. Med. Sport 2000, 3, 287–298. [Google Scholar] [CrossRef]
- Su, Y.; Elshorbagy, A.; Turner, C.; Refsum, H.; Chan, R.; Kwok, T. Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone 2019, 129, 115082. [Google Scholar] [CrossRef]
- Colucci, S.; Mori, G.; Vaira, S.; Brunetti, G.; Greco, G.; Mancini, L.; Simone, G.M.; Sardelli, F.; Koverech, A.; Zallone, A.; et al. L-carnitine and isovaleryl L-carnitine fumarate positively affect human osteoblast proliferation and differentiation in vitro. Calcif. Tissue Int. 2005, 76, 458–465. [Google Scholar] [CrossRef]
- Zhuang, T.; Liu, X.; Wang, W.; Song, J.; Zhao, L.; Ding, L.; Yang, L.; Zhou, M. Dose-Related Urinary Metabolic Alterations of a Combination of Quercetin and Resveratrol-Treated High-Fat Diet Fed Rats. Front. Pharmacol. 2021, 12, 655563. [Google Scholar] [CrossRef]
- Tomkin, G.H.; Owens, D. Obesity diabetes and the role of bile acids in metabolism. J. Transl. Intern. Med. 2016, 4, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef] [Green Version]
- Mittendorfer, B. Sexual dimorphism in human lipid metabolism. J. Nutr. 2005, 135, 681–686. [Google Scholar] [CrossRef]
- Mischke, M.; Pruis, M.G.M.; Boekschoten, M.V.; Groen, A.K.; Fitri, A.R.; van de Heijning, B.J.M.; Verkade, H.J.; Müller, M.; Plösch, T.; Steegenga, W.T. Maternal Western-style high fat diet induces sex-specific physiological and molecular changes in two-week-old mouse offspring. PLoS ONE 2013, 8, e78623. [Google Scholar] [CrossRef] [Green Version]
- Denova-Gutierrez, E.; Flores, Y.N.; Gallegos-Carrillo, K.; Ramirez-Palacios, P.; Rivera-Paredez, B.; Munoz-Aguirre, P.; Velazquez-Cruz, R.; Torres-Ibarra, L.; Meneses-Leon, J.; Mendez-Hernandez, P.; et al. Health workers cohort study: Methods and study design. Salud Publica Mex 2016, 58, 708–716. [Google Scholar] [CrossRef]
- WHO. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Available online: http://www.who.int/chp/topics/Osteoporosis.pdf (accessed on 17 March 2018).
- Tate, J.R.; Berg, K.; Couderc, R.; Dati, F.; Kostner, G.M.; Marcovina, S.M.; Rifai, N.; Sakurabayashi, I.; Steinmetz, A. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Standardization Project for the Measurement of Lipoprotein(a). Phase 2: Selection and properties of a proposed secondary reference material for lipoprotein(a). Clin. Chem. Lab. Med. 1999, 37, 949–958. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; López-Contreras, B.E.; Canizales-Quinteros, S. Gut Microbiota in Obesity and Metabolic Abnormalities: A Matter of Composition or Functionality? Arch. Med. Res. 2017, 48, 735–753. [Google Scholar] [CrossRef]






| Total | Men | Women | p-Value | |
|---|---|---|---|---|
| n = 602 | n = 145 | n = 457 | ||
| Age (years) * | 60 (50–68) | 56 (46–65) | 60 (52–68) | 0.003 |
| Age Categories, % | ||||
| <30 years | 5.2 | 8.3 | 4.2 | 0.052 |
| 30–39 years | 6.3 | 5.5 | 6.6 | 0.636 |
| 40–49 years | 11.6 | 15.9 | 10.3 | 0.067 |
| 50–59 years | 26.7 | 29.7 | 25.8 | 0.355 |
| 60–69 years | 29.6 | 24.1 | 31.3 | 0.098 |
| >70 years | 20.6 | 16.6 | 21.9 | 0.169 |
| BMI (kg/m2) * | 26.9 (24.1–30.5) | 26.5 (24.3–29.5) | 27.1 (23.8–30.8) | 0.439 |
| Nutritional Status, % | ||||
| Overweight | 39 | 41.4 | 38.3 | 0.505 |
| Obesity | 27.2 | 24.1 | 28.2 | 0.334 |
| Waist circumference (cm) * | 93 (86–100) | 97 (91–105) | 91 (84–99) | <0.001 |
| Body fat proportion * | 42.9 (37.0–47.9) | 32.3 (28.6–36.3) | 45.3 (40.5–49.8) | <0.001 |
| Leisure time physical activity (min/day) * | 12.9 (3.2–30) | 12.9 (3.2–47.1) | 12.8 (3.2–30.0) | 0.06 |
| Active (≥150/week), % | 28.7 | 32.4 | 27.6 | 0.818 |
| Missing, % | 15.9 | 15.2 | 16 | - |
| Glucose (mg/dL) * | 99 (92–109) | 101 (93–110) | 98 (91–109) | 0.162 |
| Impaired Glucose tolerance (≥100–<126 mg/dL), % | 32.1 | 35.9 | 30.9 | 0.261 |
| Type 2 diabetes, % | 18.3 | 20.7 | 17.5 | 0.385 |
| Total cholesterol (mg/dL) * | 197.5 (169–224) | 196 (162–220) | 198 (172–225) | 0.096 |
| Triglyceride (mg/dL) * | 141 (105–197) | 148 (106–207) | 138 (105–194) | 0.157 |
| HDL-C (mg/dL) * | 50.7 (42.3–59.8) | 44.6 (38.7–52.2) | 52.8 (45.2–61.9) | <0.001 |
| LDL-C (mg/dL) * | 113.1 (90.9–135.8) | 113.2 (89.4–135.9) | 113.1 (91.6–135.6) | 0.785 |
| Systolic blood pressure (mmHg) * | 120 (109–134) | 123 (114–137) | 118 (107–133) | 0.0004 |
| Diastolic blood pressure (mmHg) * | 75 (69–82) | 79 (73–85) | 74 (68–80) | <0.001 |
| Femoral neck-BMD (g/cm2) * | 0.91 (0.81–1.01) | 0.99 (0.89–1.16) | 0.88 (0.78–0.98) | <0.001 |
| Lumbar spine-BMD (g/cm2) * | 1.07 (0.95–1.18) | 1.14 (1.06–1.27) | 1.04 (0.93–1.15) | <0.001 |
| Visceral Adiposity Index * | 2.2 (1.5–3.3) | 2.0 (1.4–3.2) | 2.2 (1.5–3.4) | 0.063 |
| VAI | ||||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-Value | |
| n = 201 | n = 201 | n = 200 | ||
| Sex, % | ||||
| Women | 71.1 | 76.1 | 80.5 | 0.003 |
| Age (years) * | 58 (47–68) | 61 (51–68) | 60 (52–68) | 0.043 |
| Age Categories, % | ||||
| <30 years | 7.5 | 3.5 | 4.5 | 0.206 |
| 30–39 years | 10 | 6 | 3 | 0.005 |
| 40–49 years | 11 | 11 | 13 | 0.538 |
| 50–59 years | 26.4 | 26.9 | 27 | 0.892 |
| 60–69 years | 23.4 | 33.8 | 31.5 | 0.069 |
| >70 years | 21.9 | 18.9 | 21 | 0.083 |
| BMI (kg/m2) * | 25.2 (23.0–28.1) | 27.3 (24.5–30.8) | 28.3 (25.8–31.8) | 0.043 |
| Nutritional Status, % | ||||
| Overweight | 34.3 | 37.8 | 45 | 0.028 |
| Obesity | 18.4 | 28.9 | 34.5 | 0.0003 |
| Waist circumference (cm) * | 89 (82–96) | 94 (87–100) | 96 (88–104.5) | <0.001 |
| Body fat proportion * | 41.1 (33.3–46.1) | 43.3 (36.3–49.1) | 45.6 (38.9–49.0) | 0.0001 |
| Leisure time physical activity (min/day) * | 12.9 (3.2–42.9) | 7.7 (3.2–30.0) | 12.9 (3.2–30.0) | 0.145 |
| Active (≥150/week), % | 31.3 | 24.4 | 30.5 | 0.862 |
| Missing, % | 20.9 | 14.9 | 11.5 | 0.011 |
| Glucose (mg/dL) | 96 (88–102) | 99 (91–108) | 104 (95–121) | <0.001 |
| Impaired glucose tolerance (≥100–<126 mg/dL), % | 24.9 | 32.3 | 39 | 0.003 |
| Type 2 diabetes, % | 13.4 | 16.9 | 24.5 | 0.005 |
| Total cholesterol (mg/dL) * | 193 (163–214) | 198 (167–225) | 201 (177–236) | 0.0008 |
| Triglyceride (mg/dL) * | 95 (72–113) | 141 (121–164) | 224 (186–283) | <0.001 |
| HDL-C (mg/dL) * | 60 (52–70) | 51 (44–57) | 43 (37–50) | <0.001 |
| LDL-C (mg/dL) * | 111 (90–132) | 118 (94–141) | 112 (87–133) | 0.344 |
| Systolic blood pressure (mmHg) * | 116 (108–132) | 120 (109–133) | 122 (111–136) | 0.013 |
| Diastolic blood pressure (mmHg) * | 74 (69–80) | 74 (69–82) | 77 (70–83) | 0.014 |
| Femoral neck-BMD (g/cm2) * | 0.89 (0.80–1.02) | 0.92 (0.81–1.01) | 0.92 (0.81–1.01) | 0.209 |
| Lumbar spine-BMD (g/cm2) * | 1.08 (0.97–1.19) | 1.06 (0.95–1.17) | 1.06 (0.95–1.16) | 0.212 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-González, B.; León-Reyes, G.; Rivera-Paredez, B.; Ibarra-González, I.; Vela-Amieva, M.; Flores, Y.N.; Canizales-Quinteros, S.; Salmerón, J.; Velázquez-Cruz, R. Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population. Metabolites 2021, 11, 604. https://doi.org/10.3390/metabo11090604
Palacios-González B, León-Reyes G, Rivera-Paredez B, Ibarra-González I, Vela-Amieva M, Flores YN, Canizales-Quinteros S, Salmerón J, Velázquez-Cruz R. Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population. Metabolites. 2021; 11(9):604. https://doi.org/10.3390/metabo11090604
Chicago/Turabian StylePalacios-González, Berenice, Guadalupe León-Reyes, Berenice Rivera-Paredez, Isabel Ibarra-González, Marcela Vela-Amieva, Yvonne N. Flores, Samuel Canizales-Quinteros, Jorge Salmerón, and Rafael Velázquez-Cruz. 2021. "Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population" Metabolites 11, no. 9: 604. https://doi.org/10.3390/metabo11090604
APA StylePalacios-González, B., León-Reyes, G., Rivera-Paredez, B., Ibarra-González, I., Vela-Amieva, M., Flores, Y. N., Canizales-Quinteros, S., Salmerón, J., & Velázquez-Cruz, R. (2021). Serum Metabolite Profile Associated with Sex-Dependent Visceral Adiposity Index and Low Bone Mineral Density in a Mexican Population. Metabolites, 11(9), 604. https://doi.org/10.3390/metabo11090604