Sound Stimulation Can Affect Saccharomyces cerevisiae Growth and Production of Volatile Metabolites in Liquid Medium
Abstract
:1. Introduction
2. Results
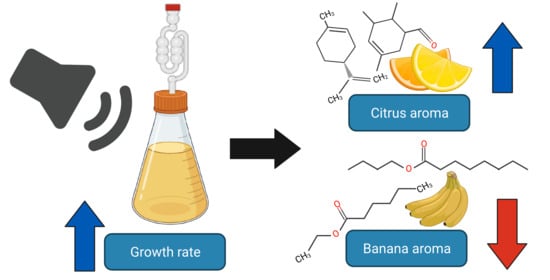
2.1. Audible Sound Affects the Growth of S. cerevisiae during Liquid Fermentation
2.2. Profile of Volatile Metabolites
3. Discussion
4. Materials and Methods
4.1. Yeast Strain
4.2. Malt Extract Micro-Fermentations
4.2.1. Sound Treatments
4.2.2. Fermentation
4.3. Analysis of Volatile Metabolites
4.3.1. Sample Preparation for Volatile Metabolite Analysis
4.3.2. Headspace Solid-Phase Microextraction (HS-SPME)
4.3.3. GC-MS Analysis
4.3.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Name of Volatile | Odour Description |
|---|---|
| Benzoic acid, methyl ester | Phenolic, dry-fruity, feijoa |
| Cyclohexanol, 1-methyl-4-(1-methylethylidene)- | Terpinol, pine |
| Limonene | Oranges |
| Benzene, (ethoxymethyl)- | Fruity pineapple |
| 7-Octen-2-ol, 2,6-dimethyl- | Lime-like, citrusy-floral |
| Benzene, 1,1′-[oxybis(methylene)]bis- | Slightly mushroom/damp |
| Isocyclocitral | Leafy citrus |
| p-Cymene | Cumin and thyme |
| (+)-4-Carene | Sweet and pungent |
| 1-Nonanol | Citronella |
| Linalool | Floral |
| Carvone | Dill, caraway or spearmint |
| cis-2-Nonene | Aroma building block |
| Camphene | Citronella or Cypress |
| Citral | Lemon |
| Benzoin | Sweet vanilla, balsam woody |
| Geraniol | Geranium flowers, coriander |
| Benzeneacetaldehyde | Honey, floral |
| Hexanoic acid, ethyl ester | Banana, pineapple (sweet fruit) |
| Phenylethyl Alcohol | Rose |
| Benzaldehyde, 2,4-dimethyl- | Almond, cherry, spicy vanilla |
| Octanoic acid, ethyl ester | Sweet apricot, banana, brandy, pear |
| Benzenepropanal, 4-(1,1-dimethylethyl)- | Bourgeonal, floral, watery |
Appendix B

References
- Heffner, H.E.; Heffner, R.S. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–22. [Google Scholar] [PubMed]
- Khait, I.; Obolski, U.; Yovel, Y.; Hadany, L. Sound perception in plants. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Mishra, R.C.; Ghosh, R.; Bae, H. Plant acoustics: In the search of a sound mechanism for sound signaling in plants. J. Exp. Bot. 2016, 67, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Sonobioreactors: Using ultrasound for enhanced microbial productivity. Trends Biotechnol. 2003, 21, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Koda, S.; Miyamoto, M.; Toma, M.; Matsuoka, T.; Maebayashi, M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500kHz. Ultrason. Sonochem. 2009, 16, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.G.; Ross, S.A. Ultrasound Increases the Rate of Bacterial Cell Growth. Biotechnol. Prog. 2003, 19, 1038–1044. [Google Scholar] [CrossRef]
- Dang, W.-M.; Wang, S.; Tian, S.-X.; Chen, B.; Sun, F.; Li, W.; Jiao, Y.; He, L.-H. Effects of infrasound on activities of 3beta hydroxysteroid dehydrogenase and acid phosphatase of polygonal cells in adrenal cortex zona fasciculate in mice. Chin. J. Ind. Hyg. Occup. Dis. 2007, 25, 91–95. [Google Scholar]
- Martirosyan, V.; Markosyan, L.; Hovhanesyan, H.; Hovnanyan, K.; Ayrapetyan, S. The frequency-dependent effect of extremely low-frequency electromagnetic field and mechanical vibration at infrasound frequency on the growth, division and motility of Escherichia coli K-12. Environmentalist 2011, 32, 157–165. [Google Scholar] [CrossRef]
- Robinson, J.M.; Cameron, R.; Parker, B. The Effects of Anthropogenic Sound and Artificial Light Exposure on Microbiomes: Ecological and Public Health Implications. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Ying, J.C.L.; Dayou, J.; Phin, C.K. Experimental Investigation on the Effects of Audible Sound to the Growth of Escherichia coli. Mod. Appl. Sci. 2009, 3, 124. [Google Scholar] [CrossRef] [Green Version]
- Shaobin, G.; Wu, Y.; Li, K.; Li, S.; Ma, S.; Wang, Q.; Wang, R. A pilot study of the effect of audible sound on the growth of Escherichia coli. Colloids Surf. B Biointerfaces 2010, 78, 367–371. [Google Scholar] [CrossRef]
- Aggio, R.B.M.; Obolonkin, V.; Villas-Bôas, S.G. Sonic vibration affects the metabolism of yeast cells growing in liquid culture: A metabolomic study. Metabolomics 2011, 8, 670–678. [Google Scholar] [CrossRef]
- Sarvaiya, N.; Kothari, V. Effect of audible sound in form of music on microbial growth and production of certain important metabolites. Microbiology 2015, 84, 227–235. [Google Scholar] [CrossRef]
- Matsuhashi, M.; Pankrushina, A.N.; Endoh, K.; Watanabe, H.; Ohshima, H.; Tobi, M.; Endo, S.; Mano, Y.; Hyodo, M.; Kaneko, T.; et al. Bacillus carboniphilus cells respond to growth-promoting physical signals from cells of homologous and heterologous bacteria. J. Gen. Appl. Microbiol. 1996, 42, 315–323. [Google Scholar] [CrossRef]
- Matsuhashi, M.; Pankrushina, A.N.L.; Takeuchi, S.; Ohshima, H.; Miyoi, H.; Endoh, K.; Murayama, K.; Watanabe, H.; Endo, S.; Tobi, M.; et al. Production of sound waves by bacterial cells and the response of bacterial cells to sound. J. Gen. Appl. Microbiol. 1998, 44, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Pelling, A.E.; Sehati, S.; Gralla, E.B.; Valentine, J.S.; Gimzewski, J.K. Local Nanomechanical Motion of the Cell Wall of Saccharomyces cerevisiae. Science 2004, 305, 1147–1150. [Google Scholar] [CrossRef]
- Edison, A.S.; Hall, R.D.; Junot, C.; Karp, P.D.; Kurland, I.J.; Mistrik, R.; Reed, L.K.; Saito, K.; Salek, R.M.; Steinbeck, C.; et al. The Time Is Right to Focus on Model Organism Metabolomes. Metabolites 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Karathia, H.; Vilaprinyo, E.; Sorribas, A.; Alves, R. Saccharomyces cerevisiae as a Model Organism: A Comparative Study. PLoS ONE 2011, 6, e16015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, e1800421. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Makhoul, S.; Aprea, E.; Romano, A.; Cappellin, L.; Jimena, A.S.; Spano, G.; Gasperi, F.; Scampicchio, M.; Biasioli, F. PTR-MS Characterization of VOCs Associated with Commercial Aromatic Bakery Yeasts of Wine and Beer Origin. Molecules 2016, 21, 483. [Google Scholar] [CrossRef]
- Steensels, J.; Meersman, E.; Snoek, T.; Saels, V.; Verstrepen, K.J. Large-Scale Selection and Breeding To Generate Industrial Yeasts with Superior Aroma Production. Appl. Environ. Microbiol. 2014, 80, 6965–6975. [Google Scholar] [CrossRef] [Green Version]
- Nagodawithana, T.W.; Steinkraus, K.H. Influence of the rate of ethanol production and accumulation on the viability of Saccharomyces cerevisiae in “rapid fermentation”. Appl. Environ. Microbiol. 1976, 31, 158–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbegal, C.; Khomenko, I.; Russo, P.; Spano, G.; Fragasso, M.; Biasioli, F.; Capozzi, V. PTR-ToF-MS for the Online Monitoring of Alcoholic Fermentation in Wine: Assessment of VOCs Variability Associated with Different Combinations of Saccharomyces/Non-Saccharomyces as a Case-Study. Fermentation 2020, 6, 55. [Google Scholar] [CrossRef]
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.-B.; Yang, B.; Wu, Y.; Li, S.-C.; Liu, W.; Duan, X.-F.; Li, M.-W. Growth and Physiological Characteristics of E. coli in Response to the Exposure of Sound Field. Pak. J. Biol. Sci. 2013, 16, 969–975. [Google Scholar] [CrossRef]
- Banerjee, S.; Goswami, A.; Datta, A.; Pyne, A.; Nikhat, A.; Ghosh, B. Effect of Different Sound Frequencies on the Growth and Antibiotic Susceptibility of Escherichia coli. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1931–1939. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.L.; Wu, W.J.; Yung, P.T. Effect of sonic stimulation on Bacillus endospore germination. FEMS Microbiol. Lett. 2016, 363, fnv217. [Google Scholar] [CrossRef] [Green Version]
- Kothari, V.; Joshi, C.; Patel, P.; Mehta, M.; Dubey, S.; Mishra, B.; Sarvaiya, N. Influence of a Mono-Frequency Sound on Bacteria can be a Function of the Sound-Level. Indian J. Sci. Technol. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavour Ingredients; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chandra, T.S.; Lekha, V.S.; Krishna, T.M. Effect of music on growth and pigment production of Brevibacterium sp. Int. J. Pharm. Chem. Biol. Sci. 2018, 8, 157–160. [Google Scholar]
- Guido, L.; Rodrigues, P.; Rodrigues, J.; Gonçalves, C.; Barros, A. The impact of the physiological condition of the pitching yeast on beer flavour stability: An industrial approach. Food Chem. 2004, 87, 187–193. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ku, H.-N.; Lin, W.-F.; Peng, H.-L.; Yew, T.-R. In Situ monitoring the effect of acoustic vibration in the form of music on the motility of Escherichia coli. Appl. Acoust. 2020, 172, 107620. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, Y.; Wu, Y. Effects of sound exposure on the growth and intracellular macromolecular synthesis of E. coli k-12. PeerJ 2016, 4, e1920. [Google Scholar] [CrossRef] [Green Version]
- Viejo, C.G.; Fuentes, S.; Torrico, D.D.; Lee, M.H.; Hu, Y.Q.; Chakraborty, S.; Dunshea, F.R. The Effect of Soundwaves on Foamability Properties and Sensory of Beers with a Machine Learning Modeling Approach. Beverages 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- De Simone, N.; Russo, P.; Tufariello, M.; Fragasso, M.; Solimando, M.; Capozzi, V.; Grieco, F.; Spano, G. Autochthonous Biological Resources for the Production of Regional Craft Beers: Exploring Possible Contributions of Cereals, Hops, Microbes, and Other Ingredients. Foods 2021, 10, 1831. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Dickinson, J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003, 3, 53–62. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Lee, S.A.; Goddard, M.R. Fungal communities are differentially affected by conventional and biodynamic agricultural management approaches in vineyard ecosystems. Agric. Ecosyst. Environ. 2017, 246, 306–313. [Google Scholar] [CrossRef]
- Wassermann, B.; Korsten, L.; Berg, G. Plant Health and Sound Vibration: Analyzing Implications of the Microbiome in Grape Wine Leaves. Pathogens 2021, 10, 63. [Google Scholar] [CrossRef]
- Rezaei, M.N.; Dornez, E.; Jacobs, P.; Parsi, A.; Verstrepen, K.; Courtin, C.M. Harvesting yeast (Saccharomyces cerevisiae) at different physiological phases significantly affects its functionality in bread dough fermentation. Food Microbiol. 2014, 39, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Stahl, G.; Ben Salem, S.N.; Chen, L.; Zhao, B.; Farabaugh, P.J. Translational Accuracy during Exponential, Postdiauxic, and Stationary Growth Phases in Saccharomyces cerevisiae. Eukaryot. Cell 2004, 3, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunge, M.; Araghipour, N.; Mikoviny, T.; Dunkl, J.; Schnitzhofer, R.; Hansel, A.; Schinner, F.; Wisthaler, A.; Margesin, R.; Märk, T.D. On-Line Monitoring of Microbial Volatile Metabolites by Proton Transfer Reaction-Mass Spectrometry. Appl. Environ. Microbiol. 2008, 74, 2179–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villas-Boas, S.; Moxley, J.F.; Åkesson, M.; Stephanopoulos, G.; Nielsen, J. High-throughput metabolic state analysis: The missing link in integrated functional genomics of yeasts. Biochem. J. 2005, 388, 669–677. [Google Scholar] [CrossRef]




| Original Source | Predicted Source | ||
|---|---|---|---|
| High-Frequency | Low-Frequency | Silence | |
| High-frequency | 6 | 0 | 0 |
| Low-frequency | 0 | 6 | 0 |
| Silence | 0 | 0 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, A.; Lindsay, M.A.; Ganley, A.R.D.; Jeffs, A.; Villas-Boas, S.G. Sound Stimulation Can Affect Saccharomyces cerevisiae Growth and Production of Volatile Metabolites in Liquid Medium. Metabolites 2021, 11, 605. https://doi.org/10.3390/metabo11090605
Harris A, Lindsay MA, Ganley ARD, Jeffs A, Villas-Boas SG. Sound Stimulation Can Affect Saccharomyces cerevisiae Growth and Production of Volatile Metabolites in Liquid Medium. Metabolites. 2021; 11(9):605. https://doi.org/10.3390/metabo11090605
Chicago/Turabian StyleHarris, Alastair, Melodie A. Lindsay, Austen R. D. Ganley, Andrew Jeffs, and Silas G. Villas-Boas. 2021. "Sound Stimulation Can Affect Saccharomyces cerevisiae Growth and Production of Volatile Metabolites in Liquid Medium" Metabolites 11, no. 9: 605. https://doi.org/10.3390/metabo11090605
APA StyleHarris, A., Lindsay, M. A., Ganley, A. R. D., Jeffs, A., & Villas-Boas, S. G. (2021). Sound Stimulation Can Affect Saccharomyces cerevisiae Growth and Production of Volatile Metabolites in Liquid Medium. Metabolites, 11(9), 605. https://doi.org/10.3390/metabo11090605