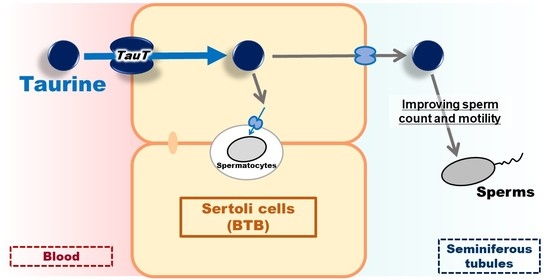
Involvement of TauT/SLC6A6 in Taurine Transport at the Blood–Testis Barrier
Abstract
:1. Introduction
2. Results
2.1. Integration Plot of [3H]taurine in Mice
2.2. Uptake of [3H]taurine by TM4 Cells
2.3. Expression Analysis of TauT in TM4 Cells
2.4. Knockdown of TauT in TM4 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents, Animals and Cells
4.2. Integration Plot Analysis
4.3. Cell Uptake Analysis
4.4. mRNA Expression Analysis
4.5. Protein Expression Analysis
4.6. Knockdown Analysis of TauT
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubo, Y.; Akanuma, S.-I.; Hosoya, K.-I. Impact of SLC6A Transporters in Physiological Taurine Transport at the Blood–Retinal Barrier and in the Liver. Biol. Pharm. Bull. 2016, 39, 1903–1911. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, B.; Dinçer, S.; Babül, A.; Duyar, I. The effect of taurine administration on vitamin C levels of several tissues in mice. Amino Acids 2003, 27, 225–228. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sil, P. Taurine provides antioxidant defense against NaF-induced cytotoxicity in murine hepatocytes. Pathophysiology 2008, 15, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H. Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem. Res. 2004, 29, 27–63. [Google Scholar] [CrossRef]
- Takatani, T.; Takahashi, K.; Uozumi, Y.; Shikata, E.; Yamamoto, Y.; Ito, T.; Matsuda, T.; Schaffer, S.; Fujio, Y.; Azuma, J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids. 2015, 47, 1549–1558. [Google Scholar]
- Yu, J.; Kim, K. Effect of taurine on antioxidant enzyme system in B16F10 melanoma cells. Adv. Exp. Med. Biol. 2009, 7, 491–499. [Google Scholar]
- Yuan, L.-Q.; Xie, H.; Luo, X.-H.; Wu, X.-P.; Zhou, H.-D.; Lu, Y.; Liao, E.-Y. Taurine transporter is expressed in osteoblasts. Amino Acids 2006, 31, 157–163. [Google Scholar] [CrossRef]
- Hayes, K.C.; Stephan, Z.F.; Sturman, J.A. Growth Depression in Taurine-Depleted Infant Monkeys. J. Nutr. 1980, 110, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Matuda, K.; Nishimura, N.; Yokogoshi, H. The effect of taurine on cholesterol degradation in mice fed a high-cholesterol diet. Life Sci. 2004, 74, 1889–1898. [Google Scholar] [CrossRef]
- Tomi, M.; Terayama, T.; Isobe, T.; Egami, F.; Morito, A.; Kurachi, M.; Ohtsuki, S.; Kang, Y.-S.; Terasaki, T.; Hosoya, K.-I. Function and regulation of taurine transport at the inner blood–retinal barrier. Microvasc. Res. 2007, 73, 100–106. [Google Scholar] [CrossRef]
- Ikeda, S.; Tachikawa, M.; Akanuma, S.-I.; Fujinawa, J.; Hosoya, K.-I. Involvement of γ-aminobutyric acid transporter 2 in the hepatic uptake of taurine in rats. Am. J. Physiol. Liver Physiol. 2012, 303, G291–G297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, R.P.; Goodman, H.; Shihabi, Z.K.; Jarow, J.P. The taurine and hypotaurine content of human semen. J. Androl. 1992, 13, 289–292. [Google Scholar] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Manna, P.; Sinha, M.; Sil, P.C. Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol. Lett. 2009, 187, 201–210. [Google Scholar] [CrossRef]
- Tsounapi, P.; Saito, M.; Dimitriadis, F.; Koukos, S.; Shimizu, S.; Satoh, K.; Takenaka, A.; Sofikitis, N. Antioxidant treatment with edaravone or taurine ameliorates diabetes-induced testicular dysfunction in the rat. Mol. Cell. Biochem. 2012, 369, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.; Gawad, H.A. Taurine dietary supplementation attenuates brain, thyroid, testicular disturbances and oxidative stress in streptozotocin-induced diabetes mellitus in male rats. Beni-Suef Univ. J. Basic Appl. Sci. 2017, 6, 247–252. [Google Scholar] [CrossRef]
- Lanzafame, F.M.; La Vignera, S.; Vicari, E.; Calogero, A. Oxidative stress and medical antioxidant treatment in male infertility. Reprod. Biomed. Online 2009, 19, 638–659. [Google Scholar] [CrossRef] [Green Version]
- Terai, K.; Horie, S.; Fukuhara, S.; Miyagawa, Y.; Kobayashi, K.; Tsujimura, A. Combination therapy with antioxidants improves total motile sperm counts: A Preliminary Study. Reprod. Med. Biol. 2019, 19, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.Y.; Mruk, D.D. The Blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2011, 64, 16–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lui, W.Y.; Mruk, D.; Lee, W.M.; Cheng, C.Y. Sertoli Cell Tight Junction Dynamics: Their Regulation During Spermatogenesis1. Biol. Reprod. 2003, 68, 1087–1097. [Google Scholar] [CrossRef]
- Kerr, J.B. Ultrastructure of the seminiferous epithelium and intertubular tissue of the human testis. J. Electron. Microsc. Tech. 1991, 19, 215–240. [Google Scholar] [CrossRef]
- Angulo, C.; Maldonado, R.; Pulgar, E.; Mancilla, H.; Cordova, A.; Villarroel, F.; Castro, M.A.; Concha, I.I. Vitamin C and oxidative stress in the seminiferous epithelium. Biol. Res. 2011, 44, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Bae, H.S.; Jin, Y.K.; Ham, S.; Kim, H.K.; Shin, H.; Cho, G.; Lee, K.J.; Lee, H.; Kim, K.M.; Koo, O.J.; et al. CRISRP/Cas9-mediated knockout of Mct8 reveals a functional involvement of Mct8 in testis and sperm development in a rat. Sci. Rep. 2020, 10, 11148. [Google Scholar] [CrossRef] [PubMed]
- Bart, J.; Hollema, H.; Groen, H.J.M.; Veries, E.G.E.; Hendrikse, N.H.; Sleijfer, D.T.; Wegman, T.D.; Vaalburg, W.; Graaf, W.T.A. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 andMRP2, in the normal blood–testis barrier and in primary testicular tumours. Eur. J. Cancer. 2004, 40, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ye, J.; Yu, J.; Chen, L.; Zhou, L.; Wang, H.; Li, Z.; Wang, C. The accumulation and efflux of lead partly depend on ATP-dependent efflux pump–multidrug resistance protein 1 and glutathione in testis Sertoli cells. Toxicol. Lett. 2014, 226, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Maeda, T.; Akaike, T.; Tamai, I. Nucleoside Transport at the Blood-Testis Barrier Studied with Primary-Cultured Sertoli Cells. J. Pharmacol. Exp. Ther. 2004, 312, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Nakada, N.; Mikami, T.; Hana, K.; Ichinoe, M.; Yanagisawa, N.; Yoshida, T.; Endou, H.; Okayasu, I. Unique and selective expression of L-amino acid transporter 1 in human tissue as well as being an aspect of oncofetal protein. Histol. Histopathol. 2013, 29, 2. [Google Scholar]
- Smith, K.; Borden, L.; Wang, C.H.; Hartig, P.R.; Branchek, T.; Weinshank, R.L. Cloning and expression of a high affinity taurine transporter from rat brain. Mol. Pharmacol. 1992, 42, 563–569. [Google Scholar]
- Liu, Q.R.; López-Corcuera, B.; Nelson, H.; Mandiyan, S.; Nelson, N. Cloning and expression of a cDNA encoding the transporter of taurine and/8-alanine in mouse brain. Proc. Natl. Acad. Sci. USA 1992, 89, 12145–12149. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; López-Corcuera, B.; Mandiyan, S.; Nelson, H.; Nelson, N. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected]. J. Biol. Chem. 1993, 268, 2106–2112. [Google Scholar] [CrossRef]
- Anderson, C.M.; Grenade, D.S.; Boll, M.; Foltz, M.; Wake, K.A.; Kennedy, D.J.; Munck, L.K.; Miyauchi, S.; Taylor, P.M.; Campbell, F.C.; et al. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: An intestinal nutrient/drug transporter in human and rat. Gastroenterology 2004, 127, 1410–1422. [Google Scholar] [CrossRef]
- Mather, J.P. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol. Reprod. 1980, 23, 243–252. [Google Scholar]
- Metzner, L.; Neubert, K.; Brandsch, M. Substrate specificity of the amino acid transporter PAT1. Amino Acids 2006, 31, 111–117. [Google Scholar] [CrossRef]
- Tachikawa, M.; Kasai, Y.; Yokoyama, R.; Fujinawa, J.; Ganapathy, V.; Terasaki, T.; Hosoya, K.-I. The blood-brain barrier transport and cerebral distribution of guanidinoacetate in rats: Involvement of creatine and taurine transporters. J. Neurochem. 2009, 111, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Vinnakota, S.; Qian, X.; Egal, H.; Sarthy, V.; Sarkar, H.K. Molecular characterization and in situ localization of a mouse retinal taurine transporter. J. Neurochem. 2002, 69, 2238–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, Y.; Obata, A.; Akanuma, S.-I.; Hosoya, K.-I. Impact of Cationic Amino Acid Transporter 1 on Blood-Retinal Barrier Transport of L-Ornithine. Investig. Opthalmology Vis. Sci. 2015, 56, 5925. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y.; Shimizu, Y.; Kusagawa, Y.; Akanuma, S.-I.; Hosoya, K.-I. Propranolol Transport Across the Inner Blood–Retinal Barrier: Potential Involvement of a Novel Organic Cation Transporter. J. Pharm. Sci. 2013, 102, 3332–3342. [Google Scholar] [CrossRef]
- Yamaoka, K.; Tanigawara, Y.; Nakagawa, T.; Uno, T. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharm. -Dyn. 1981, 4, 879–885. [Google Scholar] [CrossRef]
- Kubo, Y.; Yahata, S.; Miki, S.; Akanuma, S.-I.; Hosoya, K.-I. Blood-to-retina transport of riboflavin via RFVTs at the inner blood-retinal barrier. Drug Metab. Pharmacokinet. 2017, 32, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Haidl, G.; Schill, W.-B. Guidelines for Drug Treatment of Male Infertility. Drugs 1991, 41, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Sawamura, A.; Awata, N. Usefulness of taurine in chronic congestive heart failure and its prospective application: Current therapy of intractable heart failure. Jpn Circ. J. 1992, 56, 95–99. [Google Scholar] [CrossRef] [Green Version]





| Compounds | Percentage of Control (%) | ||
|---|---|---|---|
| Control | 100 | ± | 8 |
| Taurine | 3.70 | ± | 0.26 ** |
| β-Alanine | 4.69 | ± | 0.76 ** |
| Hypotaurine | 13.3 | ± | 3.1 ** |
| GABA | 23.2 | ± | 2.7 ** |
| GAA | 57.3 | ± | 4.2 ** |
| L-Alanine | 82.1 | ± | 2.0 |
| Probenecid | 98.7 | ± | 3.9 |
| L-Leucine | 112 | ± | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, Y.; Ishizuka, S.; Ito, T.; Yoneyama, D.; Akanuma, S.-i.; Hosoya, K.-i. Involvement of TauT/SLC6A6 in Taurine Transport at the Blood–Testis Barrier. Metabolites 2022, 12, 66. https://doi.org/10.3390/metabo12010066
Kubo Y, Ishizuka S, Ito T, Yoneyama D, Akanuma S-i, Hosoya K-i. Involvement of TauT/SLC6A6 in Taurine Transport at the Blood–Testis Barrier. Metabolites. 2022; 12(1):66. https://doi.org/10.3390/metabo12010066
Chicago/Turabian StyleKubo, Yoshiyuki, Sakiko Ishizuka, Takeru Ito, Daisuke Yoneyama, Shin-ichi Akanuma, and Ken-ichi Hosoya. 2022. "Involvement of TauT/SLC6A6 in Taurine Transport at the Blood–Testis Barrier" Metabolites 12, no. 1: 66. https://doi.org/10.3390/metabo12010066
APA StyleKubo, Y., Ishizuka, S., Ito, T., Yoneyama, D., Akanuma, S. -i., & Hosoya, K. -i. (2022). Involvement of TauT/SLC6A6 in Taurine Transport at the Blood–Testis Barrier. Metabolites, 12(1), 66. https://doi.org/10.3390/metabo12010066