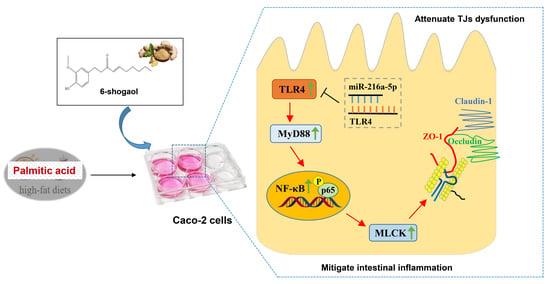
Attenuation of Palmitic Acid-Induced Intestinal Epithelial Barrier Dysfunction by 6-Shogaol in Caco-2 Cells: The Role of MiR-216a-5p/TLR4/NF-κB Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatments
2.3. Cell Viability Evaluated by the MTT Assay
2.4. Determination of Cell Permeability
2.5. Total RNA Extraction and qRT-PCR
2.6. Western Blot Analysis
2.7. Immunofluorescence Analysis
2.8. Determination of Pro-Inflammatory Cytokines by ELISA
2.9. Bioinformatics Analysis
2.10. MiR-216a-5p Transfection
2.11. Statistical Analysis
3. Results
3.1. Cytotoxicity of 6-Shogaol and PA
3.2. 6-Shogaol Alleviated PA-Induced Intestinal Barrier Dysfunction in Caco-2 Cells
3.3. 6-Shogaol Protected Caco-2 Cells against PA-Induced TJ Damage
3.4. 6-Shogaol Mitigated PA-Induced Inflammation in Caco-2 Cells
3.5. 6-Shogaol Inhibited PA-Induced Activation of the TLR4/NF-κB Signaling Pathway
3.6. MiRNA-216a-5p Mediated the Protective Effect of 6-Shogaol against TJ Damage in Caco-2 Cells
3.6.1. MiRNA-216a-5p Directly Targeted TLR4
3.6.2. MiRNA-216a-5p Was Involved in the Protection Provided by 6-Shogaol against PA-Induced TJ Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otani, T.; Furuse, M. Tight junction structure and function revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Zeng, L.; Lai, X.; Li, J.; Liu, L.; Lin, Q.; Chen, Y. Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J. Cell Mol. Med. 2018, 22, 546–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netto Candido, T.L.; Bressan, J.; Alfenas, R.D.C.G. Dysbiosis and metabolic endotoxemia induced by high-fat diet. Nutr. Hosp. 2018, 35, 1432–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Walfish, P.G. Plasma free fatty acid concentrations in obesity. N. Engl. J. Med. 1963, 268, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, L.; Wang, Y.; Peng, C.; Wu, L.; Zhang, Y.; Du, Z. High-fat diet exacerbates lead-induced blood-brain barrier disruption by disrupting tight junction integrity. Environ. Toxicol. 2021, 36, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Furet, J.P.; Debard, C.; Daira, P.; Loizon, E.; Géloën, A.; Soulage, C.O.; Simonet, C.; Lefils-Lacourtablaise, J.; Bernoud-Hubac, N.; et al. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E374–E386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.E.; Vaillant, J.C.; Oppert, J.M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]
- Ghezzal, S.; Postal, B.G.; Quevrain, E.; Brot, L.; Seksik, P.; Leturque, A.; Thenet, S.; Carriere, V. Palmitic acid damages gut epithelium integrity and initiates inflammatory cytokine production. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158530. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, H.; Li, L.; Wang, F.; Zhang, B. Shogaol potentiates sevoflurane mediated neuroprotection against ischemia/reperfusion-induced brain injury via regulating apoptotic proteins and PI3K/Akt/mTOR/s6K signalling and HIF-1α/HO-1 expression. Saudi J. Biol. Sci. 2021, 28, 5002–5010. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hsieh, M.C.; Lo, C.Y.; Liu, C.B.; Sang, S.; Ho, C.T.; Pan, M.H. 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol. Nutr. Food Res. 2010, 54, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.L.; Parkin, K.L.; Nitteranon, V.; Liang, J.; Yang, W.J.; Li, Y.; Zhang, G.D.; Hu, Q.H. Isolation of quinone reductase (QR) inducing agents from ginger rhizome and their in vitro anti-inflammatory activity. Food Res. Int. 2011, 44, 1597–1603. [Google Scholar] [CrossRef]
- Huh, E.; Choi, J.G.; Noh, D.; Yoo, H.S.; Ryu, J.; Kim, N.J.; Kim, H.; Oh, M.S. Ginger and 6-shogaol protect intestinal tight junction and enteric dopaminergic neurons against 1-methyl-4-phenyl 1,2,3,6-tetrahydropyridine in mice. Nutr. Neurosci. 2020, 23, 455–464. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Lee, I.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents TNF-α-induced barrier loss via inhibition of PI3K/Akt and NF-κB signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, A.; Rankin, C.R.; Polytarchou, C.; Lokhandwala, Z.A.; Patel, A.; Chang, L.; Pothoulakis, C.; Iliopoulos, D.; Padua, D.M. miR-24 is elevated in ulcerative colitis patients and regulates intestinal epithelial barrier function. Am. J. Pathol. 2019, 189, 1763–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Xi, W.; Li, Y.; Hai, K.; Zhou, X.; Wang, Y.; Ye, Q. MicroRNA-216a-5p in lipopolysaccharide-induced endothelial injury. Exp. Ther. Med. 2021, 22, 1426. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Wang, P.; Zhou, F. miR-489-3p inhibits TLR4/NF-κB signaling to prevent inflammation in psoriasis. Exp. Ther. Med. 2021, 22, 744. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Bhunia, A.K. Listeria monocytogenes uses Listeria adhesion protein (LAP) to promote bacterial transepithelial translocation and induces expression of LAP receptor Hsp60. Infect. Immun. 2010, 78, 5062–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najm, A.; Masson, F.M.; Preuss, P.; Georges, S.; Ory, B.; Quillard, T.; Sood, S.; Goodyear, C.S.; Veale, D.J.; Fearon, U.; et al. MicroRNA-17-5p reduces inflammation and bone erosions in mice with collagen-induced arthritis and directly targets the JAK/STAT pathway in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2020, 72, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.-Y.; Ko, H.-J.; Vallance, B.A. The intestinal epithelium: Central coordinator of mucosal immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Balda, M.S.; Matter, K. Tight junctions in health and disease. Semin. Cell Dev. Biol. 2014, 36, 147–148. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Shi, C.; Li, H.; Qu, X.; Huang, L.; Kong, C.; Qin, H.; Sun, Z.; Yan, X. High fat diet exacerbates intestinal barrier dysfunction and changes gut microbiota in intestinal-specific ACF7 knockout mice. Biomed. Pharmacother. 2019, 110, 537–545. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [Green Version]
- Cheon, H.G.; Cho, Y.S. Protection of palmitic acid-mediated lipotoxicity by arachidonic acid via channeling of palmitic acid into triglycerides in C2C12. J. Biomed. Sci. 2014, 21, 13. [Google Scholar] [CrossRef]
- Gori, M.; Altomare, A.; Cocca, S.; Solida, E.; Ribolsi, M.; Carotti, S.; Rainer, A.; Francesconi, M.; Morini, S.; Cicala, M.; et al. Palmitic acid affects intestinal epithelial barrier integrity and permeability in vitro. Antioxidants 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, D.A.; Zhang, K.; Hung, C.; Glasgow, S.; Aruni, A.W.; Unternaehrer, J.; Payne, K.J.; Langridge, W.H.R.; De Leon, M. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1β. PLoS ONE 2017, 12, e0176793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.Y.; Boivin, M.A.; Ye, D.; Pedram, A.; Said, H.M. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1β-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusugami, K.; Fukatsu, A.; Tanimoto, M.; Shinoda, M.; Haruta, J.I.; Kuroiwa, A.; Ina, K.; Kanayama, K.; Ando, T.; Matsuura, T.; et al. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Digest. Dis. Sci. 1995, 40, 949–959. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Han, X.; Uchiyama, T.; Watkins, S.K.; Yaguchi, A.; Delude, R.L.; Fink, M.P. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G621–G629. [Google Scholar] [CrossRef]
- Suzuki, T.; Hara, H. Quercetin enhances intestinal barrier function through the assembly of Zonnula Occludens-2, Occludin, and Claudin-1 and the expression of Claudin-4 in Caco-2 cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Tanabe, S.; Hara, H. Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. J. Nutr. 2011, 141, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Bai, L.; Chen, X.; Li, Y.; Qin, Y.; Meng, X.; Zhang, Q. 6-Shogaol ameliorates diabetic nephropathy through anti-inflammatory, hyperlipidemic, anti-oxidative activity in db/db mice. Biomed. Pharmacother. 2018, 97, 633–641. [Google Scholar] [CrossRef]
- Ha, S.K.; Moon, E.; Ju, M.S.; Kim, D.H.; Ryu, J.H.; Oh, M.S.; Kim, S.Y. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuroprotection. Neuropharmacology 2012, 63, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Kont, I.; Primke, T.; Niebergall, L.S.; Zech, T.; Fürst, R. Ginger constituent 6-shogaol inhibits inflammation- and angiogenesis-related cell functions in primary human endothelial cells. Front. Pharmacol. 2022, 13, 844767. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, B.; Fu, J.; Zhu, X.; Song, E.; Song, Y. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-κB signaling pathway in HUVEC cells. J. Hazard. Mater. 2021, 404, 124050. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Ahn, S.I.; Lee, J.K.; Youn, H.S. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol. Cells 2009, 27, 211–215. [Google Scholar] [CrossRef]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Moriez, R.; Salvador-Cartier, C.; Theodorou, V.; Fioramonti, J.; Eutamene, H.; Bueno, L. Myosin light chain kinase is involved in lipopolysaccharide-induced disruption of colonic epithelial barrier and bacterial translocation in rats. Am. J. Pathol. 2005, 167, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.Y.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zeng, H.; Lei, L.; Tong, X.; Yang, L.; Yang, Y.; Li, S.; Zhou, Y.; Luo, L.; Huang, J.; et al. Tight junctions and their regulation by non-coding RNAs. Int. J. Biol. Sci. 2021, 17, 712–727. [Google Scholar] [CrossRef]
- Liu, Z.; Li, C.; Chen, S.; Lin, H.; Zhao, H.; Liu, M.; Weng, J.; Liu, T.; Li, X.; Lei, C.; et al. MicroRNA-21 increases the expression level of occludin through regulating ROCK1 in prevention of intestinal barrier dysfunction. J. Cell. Biochem. 2019, 120, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Haines, R.J.; Beard, R.S., Jr.; Eitner, R.A.; Chen, L.; Wu, M.H. TNFα/IFNγ mediated intestinal epithelial barrier dysfunction is attenuated by microRNA-93 downregulation of PTK6 in mouse colonic epithelial cells. PLoS ONE 2016, 11, e0154351. [Google Scholar] [CrossRef] [PubMed]









| Genes | Primer | Sequence |
|---|---|---|
| Claudin-1 | Forward | CACCGTCTGTGTTTGAGCA |
| Reverse | CAAACCACCGCTTACAGATG | |
| Occludin | Forward | GACTATGTGGAAAGAGTTGAC |
| Reverse | ACCGCTGCTGTAACGAG | |
| ZO-1 | Forward | TTCACGCAGTTACGAGCAAG |
| Reverse | TTGGTGTTTGAAGGCAGAGC | |
| IL-6 | Forward | ACTCACCTCTTCAGAACGAATTG |
| Reverse | CCATCTTTGGAAGGTTCAGGTTG | |
| IL-1β | Forward | AGCTACGAATCTCCGACCAC |
| Reverse | CGTTATCCCATGTGTCGAAGAA | |
| TNF-α | Forward | GGCAGTCAGATCATCTTCTCGAA |
| Reverse | TGAAGAGGACCTGGGAGTAGATG | |
| GAPDH | Forward | CTCCTCCTGTTCGACAGTCA |
| Reverse | CGACCAAATCCGTTGACTCC | |
| U6 | Forward | GGAACGATACAGAGAAGATTAGC |
| Reverse | TGGAACGCTTCACGAATTTGCG | |
| MiR-140-5p | Forward | GCGCAGTGGTTTTACCCTATGGTAG |
| MiR-145-5p | Forward | GTCCAGTTTTCCCAGGAATCCCT |
| MiR-506-3p | Forward | CGCTAAGGCACCCTTCTGAGTAGA |
| MiR-216a-5p | Forward | CGTAATCTCAGCTGGCAACTGTGA |
| MiR-489-3p | Forward | CCGTGACATCACATATACGGCAGC |
| MiR-520b-3p | Forward | CCGCAAAGTGCTTCCTTTTAGAGGG |
| MiR-302b-3p | Forward | CGCCGTAAGTGCTTCCATGTTTTAGT |
| MiR-373-3p | Forward | GGAAGTGCTTCGATTTTGGGGTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, F.; Li, B.; Wang, Y.; Xu, L.; Li, D.; Li, F.; Sun-Waterhouse, D. Attenuation of Palmitic Acid-Induced Intestinal Epithelial Barrier Dysfunction by 6-Shogaol in Caco-2 Cells: The Role of MiR-216a-5p/TLR4/NF-κB Axis. Metabolites 2022, 12, 1028. https://doi.org/10.3390/metabo12111028
Ouyang F, Li B, Wang Y, Xu L, Li D, Li F, Sun-Waterhouse D. Attenuation of Palmitic Acid-Induced Intestinal Epithelial Barrier Dysfunction by 6-Shogaol in Caco-2 Cells: The Role of MiR-216a-5p/TLR4/NF-κB Axis. Metabolites. 2022; 12(11):1028. https://doi.org/10.3390/metabo12111028
Chicago/Turabian StyleOuyang, Fangxin, Bo Li, Yuli Wang, Longhua Xu, Dapeng Li, Feng Li, and Dongxiao Sun-Waterhouse. 2022. "Attenuation of Palmitic Acid-Induced Intestinal Epithelial Barrier Dysfunction by 6-Shogaol in Caco-2 Cells: The Role of MiR-216a-5p/TLR4/NF-κB Axis" Metabolites 12, no. 11: 1028. https://doi.org/10.3390/metabo12111028
APA StyleOuyang, F., Li, B., Wang, Y., Xu, L., Li, D., Li, F., & Sun-Waterhouse, D. (2022). Attenuation of Palmitic Acid-Induced Intestinal Epithelial Barrier Dysfunction by 6-Shogaol in Caco-2 Cells: The Role of MiR-216a-5p/TLR4/NF-κB Axis. Metabolites, 12(11), 1028. https://doi.org/10.3390/metabo12111028